Action Point 8 - Strategy to improve the use and access to reference standards
Last update: 04/07/2024
Background
During the meeting of WG1 Quality in January 2022, the Member States highlighted the challenges they face in accessing reference standards, including for COVID-19, which is critical to test the quality of the products on the market. These concerns were driven by the lack of existing reference standards for some products (e.g. COVID-19 vaccines), but also difficulties in accessing reference standards due to insufficient information about possible sources, challenges in producing reference standards, and the high costs associated with procurement and supply (including transport).
To address this need, the Assembly created in 2022 a dedicated action point (AP8) in the SEARN workplan, led by Working Group 1 (WG1) Quality.
Scope and definitions
General
A reference material is a critical reagent in the developmental cycle of several medicinal products. Among other things, it ensures the products are of high quality and safe. In this document, both chemical and biological reference materials are described. As per the WHO General guidelines for the establishment, maintenance and distribution of chemical reference substances, a chemical reference substance is an ‘authenticated, uniform material that is intended for use in specified chemical and physical tests, in which its properties are compared with those of the product under examination, and which possesses a degree of purity adequate for its intended use’ (1). The same guideline distinguishes:
- Primary chemical reference substance, widely acknowledged to have the appropriate qualities within a specified context, and whose assigned content when used as an assay standard is accepted without requiring comparison with another chemical substance.
- Secondary chemical reference substance, a substance whose characteristics are assigned and/or calibrated by comparison with a primary chemical reference substance.
- Certified reference material (CRS): reference material characterized by a metrologically valid procedure for one or more specified properties, accompanied by a certificate that provides the value of the specified property, its associated uncertainty and a statement of metrological traceability.
- International Chemical Reference Substance, primary chemical reference substances established on the advice of the WHO Expert Committee on Specifications for Pharmaceutical Preparations and supplied primarily for use in physical and chemical tests and assays described in the specifications for quality control of drugs published in The International Pharmacopoeia or proposed in draft monographs.
- Pharmacopeial reference standards, established and distributed by pharmacopeial authorities following the general principles of the ISO Guide: General requirements for the competence of reference material producers (ISO 17034:2016).
Further, the WHO Recommendations for the preparation, characterization and establishment of international and other biological reference standards also identify biological reference standards which are critical for biological medicines and in vitro diagnostics (IVD) (2). Biological reference standards usually comprise materials of complex composition that require biological or immunological assay for appropriate characterization, and are intended to facilitate standardized characterization of biological samples, whatever the type of measurement or method used.
Biological Reference Standards
Biological reference standards have many applications. This material (reagent) can be used to establish a well-defined parameter for assessing safety, efficacy, and quality of IVDs and vaccines. Typically, biological reference materials are generated from convalescent patient samples (blood, plasma, serum, etc.) as they would have shown clinical symptoms of a target disease (in some cases animal model sources are used). Several analytes such as antigens (antibodies and nucleic acids) and/or toxins secreted by the causative agent are assessed in the production of this reagent. Biological reference material can further be refined into international reference standard.
International reference standard (IS) is the primary calibrant and of the highest order. IS are solely established by the WHO Expert Committee on Biological Standardization. Establishment of a WHO IS follows a collaborative study involving various users of the material (including national control laboratories, IVD manufacturers and other accredited laboratories) and as many different, well-established assays as feasible. The material used should resemble as closely as possible the natural analyte of the clinical sample to be measured. An assessment of commutability should be performed as part of the collaborative study where appropriate and feasible. By definition, an IS has a specified value expressed in International Units (IU). This value is arbitrarily assigned based on the results of the collaborative study. WHO IS should not be used for more routine procedures such as validation of assays and as run controls.
Secondary standards (Regional or national reference materials, laboratory or manufacturer’s working calibrator), which is calibrated against the IS. The titre, composition and method of production of secondary standards will vary but should be suitable for obtaining sufficient measurements, when dilution is needed, to achieve an accurate calibration. Regardless of the method of production, each calibration will have a stated measurement uncertainty.
Tertiary standards (external control materials, working reagents or standards, manufacturer’s product calibrator), which is calibrated against the secondary standards. The standard may be formulated from either biological (for example, patient-derived) or non-biological material. However, regardless of the material used, all references in the traceability chain should also demonstrate commutability to the clinical sample of the tested analyte. Tertiary standards are typically formulated as a liquid preparation and may comprise a concentration of the analyte that is detected without dilution in the linear range of the assay it is intended for. They will often be used as an external control material in addition to that normally supplied by the assay manufacturer. Regular monitoring of such material may allow for the early detection of problems with assay performance.
The behaviour of the reference standard should resemble as closely as possible the behaviour of test samples in the assay systems used to test them. As per WHO, ‘The concept of commutability seeks to establish the extent to which the reference standard is suitable to serve as a standard for the variety of samples being assayed’. (TRS 943 - Annex 3: WHO general guidelines for the establishment, maintenance and distribution of chemical reference substances).
General objective
To improve access and use of reference standards in the region.
Guiding framework for the optimal use of reference standards
A Regional hybrid workshop on the optimal use of reference standards was conducted from 14-15 May 2024 in Bangkok, Thailand.
The conclusions of the meeting, endorsed by the Assembly of SEARN, have defined a vision for the regional collaboration between NQCLs:
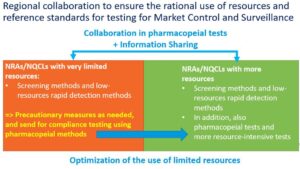
Considering this vision, a set of recommendations were developed, defining a guiding framework for SEARN, WHO and partners which addresses:
- Compliance with good practices and strengthening QMS
- Optimal use of reference standards
- Coordination with other initiatives beyond SEARN.
The details of these recommendations are provided in the highlights from the meeting:
Reducing the use of reference standards
During the 2022-2023 work plan, SEARN also reflected on strategies and good practices to reduce the use of reference standards.
Indeed, procuring reference standards may be difficult in certain areas of the world due to delays in their delivery and the cost of purchase. The number of reference substances prescribed in authoritative analytical procedures shall therefore be reduced, if possible.
Acknowledging this situation, The International Pharmacopoeia applies, for example, the following strategies and practices when elaborating monographs:
- in situ preparation of impurities for identification of related substances/impurities;
- establishment of compounded reference substances that contain several potential impurities for identification;
- quantification of impurities by comparing their detector responses with the response of the parent compound in a diluted sample solution along with the establishment of correction factors to compensate for differences in the responses of the impurity and the parent compound;
- provision of International Infrared Reference Spectra (IIRS) for use in identification tests;
- provision of assay methods not requiring reference substances, like titrations and ultraviolet spectrophotometry using absorptivity values. These methods shall be provided as alternatives in particular to chromatographic assays in monographs for pharmaceutical substances.
These strategies are only be applied when, during the elaboration of the methods, evidence has been obtained demonstrating that the intended measures do not compromise the quality of the analytical results or the ability of the tests to conclusively demonstrate conformance to the applicable standards.
Regional mechanism to list suppliers of reference standards
WG1 Quality was charged with establishing and maintaining a list of trusted suppliers of reference standards. The main mechanism to list suppliers will be reliance (established suppliers, such as pharmacopoeias).
When needed, a regional mechanism for the evaluation of suppliers of reference standards was also developed. WG1 will be responsible for the assessment of new vendors, as well as inspection and testing as required. Based on the assessment, WG1 will provide a recommendation on the inclusion of the new vendor in the regional list.
Reliance on reliable sources of reference standards
As preparation to the list of trusted suppliers of reference standards, SEARN has identified reliable sources of reference standards for chemical and biological reference standards.
Reliable sources of Chemical Reference Standards
The following possible reliable sources of chemical reference standards sources for SEARN NQCLs were identified:
| Organization name | Country | Type |
|---|---|---|
| USP | United States | Primary Reference Standards |
| BP | United Kingdom | Primary Reference Standards |
| Ph.Eur. | France | Primary Reference Standards |
| WHO (Ph.Int.) | France | Primary Reference Standards |
| IPC | India | Primary Reference Standards |
| ASEAN | Thailand | Secondary Reference Standards |
| INCQS | Brazil | Secondary Reference Standards |
| DMSc | Thailand | Secondary Reference Standards |
| NQCLDF | Indonesia | Secondary Reference Standards |
Further links are provided on the WHO website to webpages of pharmacopoeias that provide information on reference substances established to support the performance of analytical provisions described in the respective pharmacopoeias:
Reliable sources of Biological Reference Standards
The following possible reliable sources of biological reference standards sources for SEARN NQCLs were identified:
| Organization name | Country | Type |
|---|---|---|
| Centers for Disease Control and Prevention (CDC) | United States | WHO Biological Reference Standards – custodian laboratories |
| European Directorate for the Quality of Medicines and Healthcare (EDQM) | France | WHO Biological Reference Standards – custodian laboratories |
| National Institute of Allergy and Infectious Diseases (NIAID) | United States | WHO Biological Reference Standards – custodian laboratories |
| National Institute of Biological Standards and Control (NIBSC) | United Kingdom | WHO Biological Reference Standards – custodian laboratories |
| North West Lipid Research Laboratories | United States | WHO Biological Reference Standards – custodian laboratories |
| Paul Ehrlich Institute | Germany | WHO Biological Reference Standards – custodian laboratories |
| Paul Ehrlich Institute | Germany | Secondary reference standards |
| BioQControl | Netherlands | Secondary Reference Standards |
| National Serology Reference Laboratory | Australia | Secondary Reference Standards |
| Royal College of Pathologists of Australasia Quality Assurance Programs (RCPAQAP) | Australia | Secondary reference standards |
Regional mechanism to facilitate sharing and testing of samples between the SEARN NQCLs
The possibility of sharing samples between NQCLs is a tool to strengthen the individual capacity for testing in each of the member states, as well as to strengthen the network in terms of a consolidated approach to testing.
WG1 will work towards a system based on:
- Information sharing on testing capacities relying on a simple database (e.g. shared excel spreadsheet), in which information should be regularly updated by each NQCL
- And a system to trigger regional testing
- ad hoc testing (e.g. confirmatory test for EG/DEG): based on a call for expression of interest and bidding
- systematic testing as part of regional programme
Worksharing: other means to improve access to reference standards
SEARN identified other valid approaches which could be implemented in order to improve NQCLs access to reference standards, other than purchasing them directly to suppliers, such as WHO, USP, BP, EP or Indian Pharmacopoeia. The following options are two approaches which may be implemented regionally in order to overcome this issue.
Approach 1: Establishing specific procurement contracts with an established and duly qualified Reference Standards Supplier
- Needs Assessment and Alignment: Conduct a needs assessment of the regional NQCLs to identify the specific reference standards required. This survey should be held on a periodic basis, such as annual, biannual or trienal.
- Supplier Identification and Evaluation: Identify an established supplier, with a comprehensive range of reference standards (primary, secondary, working, certified reference material). Evaluate based on their reputation, product quality, compliance with international standards, and ability to meet the regions’ requirements, ensuring alignment between the identified needs and capabilities of the potential supplier(s), and qualify the supplier.
- Contract/MoU Negotiation: Initiate discussions with the chosen reference standards supplier to negotiate the terms of collaboration/contractual terms, keeping in mind the type of standards, the expected number of necessary units (to be able to determine a minimum stock per standard), special transport conditions, expiry dates, among other items.
- Documentation and Legal Framework: Draft a comprehensive Contract/MoU that outlines the terms and conditions of the collaboration. Important clauses to include, intellectual property rights, confidentiality, liability, dispute resolution, and termination procedures.
- Infrastructure and integration: Establish necessary infrastructure to integrate the reference standards from supplier into the regional network of NQCLs, such as a centralized database/digital platform to manage inventory. An additional option could be to get the supplier to do this as well.
- Logistics and Distribution: ensure that logistics and distribution protocols, with suitable transport validation, are implemented by the supplier, to ensure timely and efficient delivery from supplier to NQCLs.
- Quality Assurance and Monitoring: Implement a QA program to verify integrity and compliance of the reference standards received from supplier.
- Review and Continuous improvement: Regularly review the effectiveness of the collaboration and the satisfaction of the NQCLs with reference standards provided, namely thru supplier requalification. Seek feedback from NQCLs to identify areas of improvement and make necessary adjustments to the collaboration.
Approach 2: Establishing a Central Repository for Reference Standards (CRRS)
- Needs Assessment and Alignment: : Conduct comprehensive needs assessment to determine specific requirements and standards needed by NQCLs in the region. This survey should be held on a periodic basis, such as annual, biannual or trienal.
- Resource planning: Allocate resources for establishing the CRRS, namely financial, structural and human resources.
- Supplier Identification: Research and identify potential suppliers with a comprehensive range of reference standards (primary, secondary, working, certified reference material).
- Supplier evaluation: Evaluate the supplier(s) based on their reputation, product quality, compliance with international standards, and ability to meet the regions’ requirements, ensuring alignment between the identified needs and capabilities of the potential supplier(s), and qualify the supplier(s).
- Technical Agreement/MoU Negotiation: Develop a comprehensive technical agreement/MoU outlining terms of collaboration with the chosen reference standards supplier(s). Include aspects such as supply agreements, pricing, quality control, delivery schedules, return policies and dispute resolution mechanisms.
- Infrastructure Setup: Establish the necessary infrastructure for the CRRS, including storage facilities, equipment for handling and cataloging reference standards, an inventory management system to track, stock/availability and expiry dates of reference standards, as well as human resources needed to manage the CRRS.
- Procurement and Inventory Management: Procure the required standards based on needs assessment and update the inventory.
- Training and Documentation: Provide training to staff members responsible for managing the CRRS on proper storage, handling, and documentation procedures.
Next steps
- Support the development and implementation of a system to facilitate sharing and testing of samples between the SEARN NQCLs
- Support the finalisation and implementation of the expansion of the composition of WG1 (as needed) to support listing of suppliers of references standards and, if needed, their assessment
- To develop a strategy to ensure alignment between NQCL needs for primary reference standards and certified reference materials (CRM) and established regional and international producers and pharmacopoeias, collect and share information about official reference standards access programmes, and support convergence on the definitions of the different categories of reference standards
- To support Members in their risk-based approaches through identifying minimum standards for screening strategies
- To further develop the strategy for biological references standards
References
- Avumegah, M.S., Mattiuzzo, G., Särnefält, A. et al. Availability and use of Standards in vaccine development. npj Vaccines 8, 95 (2023). https://doi.org/10.1038/s41541-023-00692-0
- General guidelines for the establishment, maintenance and distribution of chemical reference substances - TRS 943 - Annex 3 [Internet]. 2007. Available from: https://www.who.int/docs/default-source/medicines/norms-and-standards/guidelines/quality-control/trs943-annex3-establishmentmaintenance-distribution-chemica-reference-substances.pdf?sfvrsn=71064286_0
- Recommendations for the preparation, characterization and establishment of international and other biological reference standards, Annex 2, TRS No 932 [Internet]. 2004. Available from: https://www.who.int/publications/m/item/annex2-trs932
- WHO manual for the preparation of secondary reference materials for in vitro diagnostic assays designed for infectious disease nucleic acid or antigen detection: calibration to WHO International Standards, Annex 6, TRS No 1004. https://cdn.who.int/media/docs/default-source/biologicals/blood-products/document- migration/secstandmanwho_trs_1004_web_annex_6.pdf?sfvrsn=d6937af8_3&download=true
