The South-East Asia Regulatory Network (SEARN) is a volunteer association of the National Regulatory Authorities of the countries in the WHO South-East Asia region: Bangladesh, Bhutan, the Democratic People's Republic of Korea, India, Indonesia, Maldives, Myanmar, Nepal, Sri Lanka, Thailand and Timor-Leste.
Vision: To support timely access to affordable medical products of assured quality, safety and efficacy in all countries of the South-East Asia region and beyond.
Mission: To develop and strengthen regulatory collaboration, convergence and reliance in the South-East Asia region over shared regulatory issues and challenges, that will build trust and capacity, and will enable National Regulatory Authorities (NRAs) to fulfil their mandates and better safeguard public health.
Objectives:
- Information sharing: Create an enabling environment to enhance communication and information sharing on regulatory policies, guidelines, standards, procedures, outputs and regulated products and entities between NRAs in the region.
- Systems strengthening: Facilitate and support regulatory capacity development to enhance regulatory skills and competencies and strengthen regulatory systems in the region.
- Convergence: Promote convergence and alignment of regulatory approaches and requirements based on international standards and good regulatory practices.
- Collaboration: Identify and develop potential work sharing and reliance processes to help address common work areas and optimize use of existing regulatory capacities and expertise available in the region.
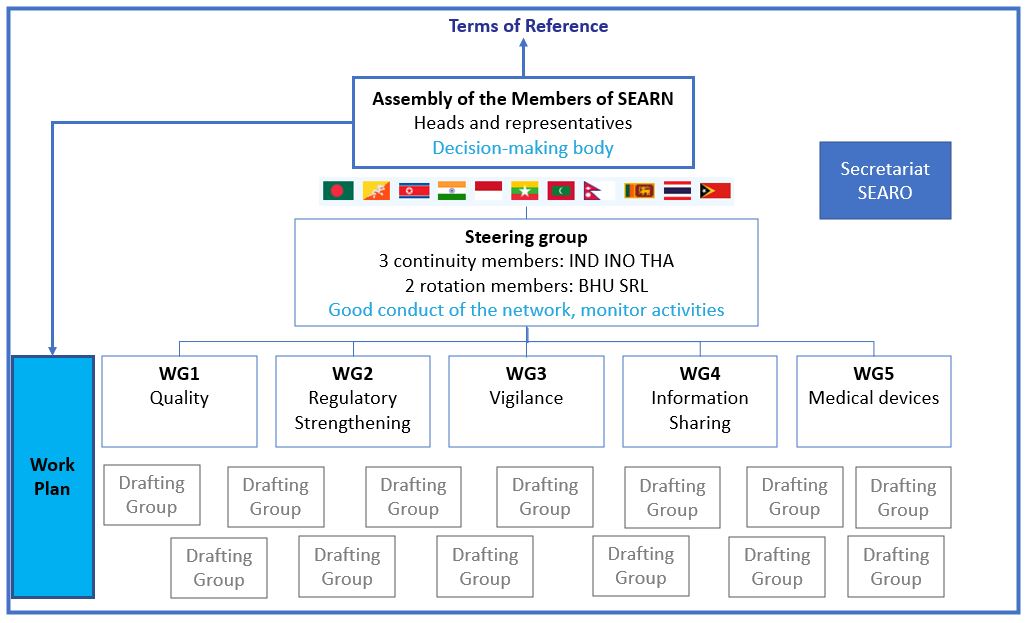
Terms of reference
The latest version of the terms of reference of the Network (v.7) was adopted during the SEARN Assembly in July 2024:
The general organization of the SEARN is summarised in the adjacent diagramme: