Action Point 12 - Ensure preparedness against public health emergencies
Last update: 04/07/2024
Background
During the meeting of Working Group 4 (WG4) Information Sharing in January 2022, the Member States highlighted the challenges they faced during the COVID-19 pandemics, including identifying relevant information among all available information.
In time of public health emergency, the National Regulatory Authorities (NRA) play a key role in ensuring timely access to quality, safe and effective countermeasures (including medicines, vaccines, medical devices and diagnostics). In such context, they are an important part of a complex international and national ecosystem of organisations, with various scopes and responsibilities, and which all need to closely cooperate to ensure an optimal response. At the international level, the environment is mostly framed by the International Health Regulations (IHR) which is coordinated by the WHO. At the national level, the Ministry of Health usually coordinate the activities of other actors, and usually have their own intelligence for response protocols and activities. Often, an Incident management/command system is set up, in which the NRA is included. Further, most countries also have a Health Research Council which often leads on the generation of evidence (at the international level or for country-specific data). National health authorities/council, professional associations, learned societies, are usually provided with the mandate to develop and approve clinical guidelines. Finally, throughout their work, the NRAs need to actively liaise, request and review information from the manufacturers, marketing authorisation holders and Clinical Research Organisations, among other actors, as per their legal mandate and provisions.
To address this need, the Assembly created in 2022 a dedicated action point (AP12) in the SEARN workplan, led by Working Group 4 (WG4) Information Sharing.
Scope and definitions
The WHO has defined an infodemic as ‘too much information including false or misleading information in digital and physical environments during a disease outbreak’, which may be expanded in the scope of this work to any public health emergency (1).
While countries may have their own definitions of a public health emergency, WHO has defined an emergency as ‘a situation impacting the lives and well-being of a large number of people or a significant percentage of a population and requiring substantial multi-sectoral assistance’(2). Further, the 2005 International Health Regulations (IHR) defines public health emergency of international concern as “an extraordinary event which is determined to constitute a public health risk to other States through the international spread of disease and to potentially require a coordinated international response”, which implies that “a situation that is serious, sudden, unusual or unexpected; carries implications for public health beyond the affected State’s national border; and may require immediate international action” (3).
During public health emergencies, National Regulatory Authorities (NRAs) participate in the detection and response to false and misleading information, for which the WHO developed a strategy and a set of tools which can be accessed on its website: https://www.who.int/health-topics/infodemic#tab=tab_1
However, the NRAs also faced challenges during COVID-19 in identifying the most relevant information among the huge amount of information available, including when only considering true /correct information. This difficulty was further aggravated in a context where NRAs human resources were heavily burdened by multiple and urgent tasks which were required to ensure timely access to quality, safe and effective medical products.
Limited resources are available to support NRAs in identifying relevant information in such time, which is the focus of this strategy.
General objective
To ensure preparedness against public health emergencies.
Regional mechanism of coordinated response to a situation of concern or a public health emergency
SEARN established a regional mechanism enabling the network to trigger a coordinated response to a situation of concern or a public health emergency, complementing the international health regulation:
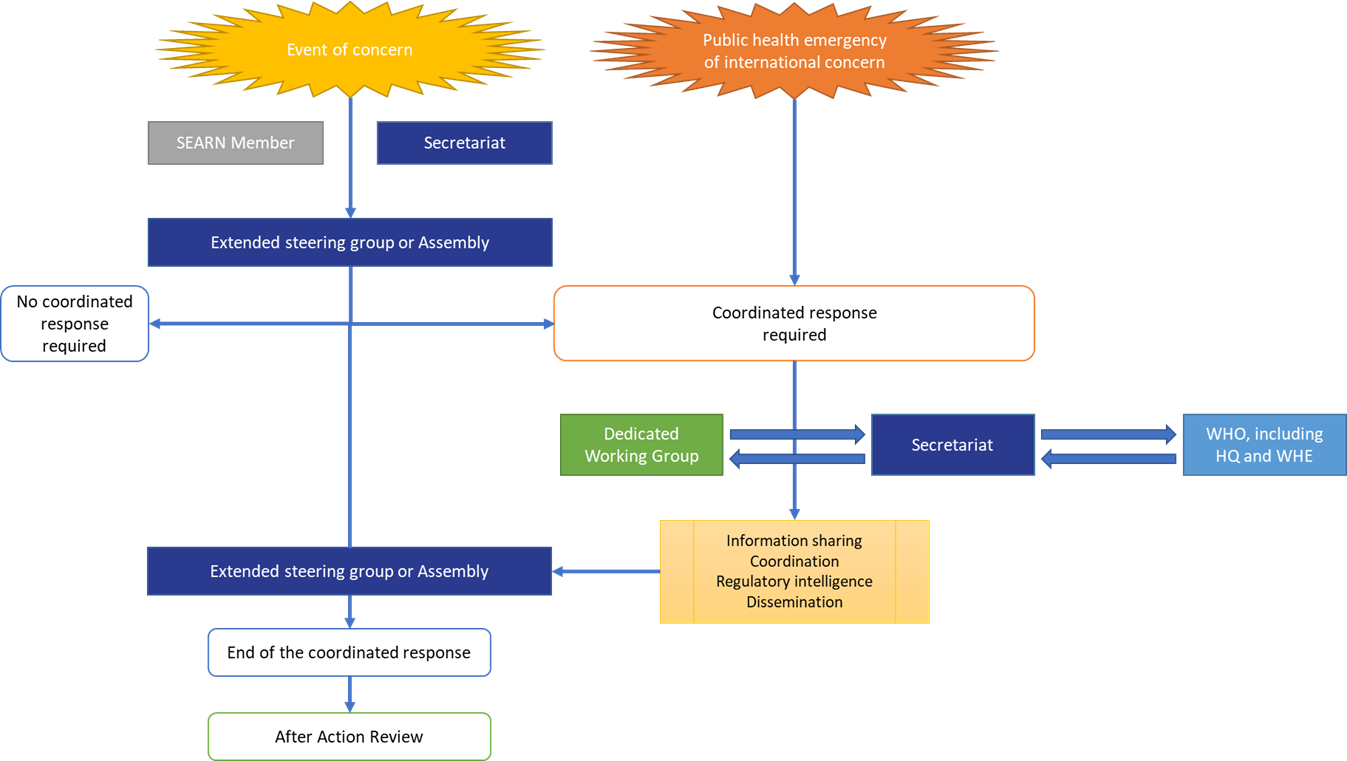
Events which may require a coordinated response from SEARN
Whenever a signal or event of concern is identified, especially when international spread is expected, the SEARN member should first contact its national focal point for International Health Regulation, with assistance from the Ministry of Health as needed.
The Network identified that the following events may require a coordinated response from SEARN:
- Public health emergency in which at least two Members of SEARN are implicated;
- Public health emergency in other countries which may affect the region;
- Emerging infectious disease in any part of the world;
- To combat misleading and false information about infectious disease outbreaks;
- Health emergencies due to substandard or falsified medicines in one country;
- Disease outbreak concerning at least two Members of SEARN
In addition, triage is also required to identify only those situations relevant for NRAs, i.e. situations which require a specific action from the NRA regarding any product in the scope of SEARN, such as:
- Authorisation (e.g. EUA, clinical trials, imports, also considering medical devices)
- Facilitating/monitoring availability,
- Vigilance/market surveillance
- Communication.
When a SEARN Member (or the secretariat) identifies such event which may require a coordinated response from SEARN, the dedicated template, available on the SEARN internal platform, is filled and shared with the secretariat.
The secretariat shares the template with the WHO Health Emergencies Programme (WHE) department to confirm the risk assessment. WHE provides a response without delay which may include a request for further information to support risk assessment (in accordance with International Health Regulation, if applicable). Further actions are taken as per the risk analysis:
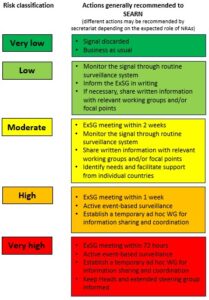
ExSG: Extended Steering Group
The results of the analysis of the event are shared without delay and within 24 hours in writing with the extended steering group, together with the proposed action(s).
At any time, and regardless of the results of the risk analysis, one SEARN member or the secretariat may still request the organisation of an urgent meeting of the extended steering group.
Preparedness and initiation of a SEARN coordinated response to a public health emergency
Any SEARN Member or the secretariat can request the Network to consider initiating a SEARN coordinated response to a public health emergency when an event of concern arises. Such requests may also provide an opportunity to share and verify with the Network any information from the Member of SEARN related to an event of concern which may become a public health emergency.
The decision should be taken by the Steering group, in a formation extended to any other interested Member States, or by the Assembly of the Members of SEARN, or automatically when a Public health emergency of international concern is declared by WHO.
If SEARN agrees that an event of concern requires a coordinated response from the Network, a dedicated and temporary working group may be created. The dedicated working group discusses the initial regulatory intelligence strategy and changes in this strategy when required, as well as any other matter related to the SEARN response to this public health emergency. The regulatory intelligence strategy should define the scope (e.g. which products to focus on, from which sources of information, including medical devices, and standards). The strategy should also define which documents should be recorded/reference on the internal platform for ease of access.
First meeting of the extended steering group
When required, a meeting of the extended steering group is organised by the secretariat as per the outcome of the risk analysis. When an urgent meeting is required, the secretariat may follow up with members through any communication means.
During the meeting, the Member (or secretariat) who raised the issue will be invited to make a presentation. The secretariat, supported by WHE, will present the results of the analysis of the risk, to support the discussions of the extended steering group.
Especially, the following decisions are expected from the first meeting:
- Definition of the initial needs of SEARN members
- Agreed course of actions
- Need to establish a temporary ad hoc working group for information sharing and coordination within SEARN
- Need for regulatory intelligence and, if needed, initial strategy (key words, confirm key targeted sources of information)
- Any other actions (e.g. information sharing, coordinated communication between NRAs, coordination with other regional or international groups, etc)
Decisions are adopted by consensus, or in the absence of the latter, by a majority vote of the participating countries. If there is no majority during the final vote, the Chair of the steering group has the final vote.
First meeting of the dedicated working group
If the extended steering group considers that an event requires coordination from SEARN, a dedicated working group may be created. By default, it is constituted of the SEARN focal points, who may be complimented or replaced by other nominated members.
The SEARN secretariat coordinates with the resources required within and beyond WHO.
Especially, the following items are expected during the first meeting:
- Briefing
- Review of the needs of SEARN members
- Discuss regulatory intelligence strategy (key words, confirm key targeted sources of information)
- Discuss information to be shared between SEARN NRAs
- Discuss needs and potential actions
Agree on date of the next meeting
Following meetings of the dedicated working group
The following meetings are organised as agreed during the previous meetings.
Regulatory intelligence efforts during public health emergency
When SEARN decides that regulatory intelligence for public health emergency is required, the Secretariat will:
- Encourage Member States to strengthen their Event-Based Surveillance system and facilitate information sharing between Member States
- Disseminate relevant information from WHO headquarters
- Work in collaboration with the WHO Health Emergencies Programme (WHE) to identify resources to use the EIOS platform as per the regulatory intelligence strategy defined by the dedicated working group
- Support easy access to procedures to facilitate supply/donations/support from national and international stakeholders
Communication of information
- To facilitate communication and sharing of information, the following mechanisms will be considered:
- Regular meeting of the dedicated working group
- Workshops on new guidelines/standards and their implementation
- Mailing lists
- Dedicated database / discussions on the SEARN internal platform
A dedicated folder on the SEARN internal platform for ease of access to key documents, as defined in the strategy.
End of the SEARN coordinated response to a public health emergency and lessons learned
The Steering group, in a formation extended to any other interest Member States or the Assembly of the Members of SEARN can decide at any time to end totality or parts of the coordinated response to a public health emergency.
The end of the coordinated response to a public health emergency will be systematically followed by an exercise of After Action Review to document the lessons from the experience and further strengthen the strategy.
Regulatory intelligence in time of public health emergency
Public Health Intelligence
Public Health Intelligence (PHI) is defined by WHO as “…a core public health function responsible for identifying, collecting, connecting, synthesizing, analyzing, assessing, interpreting and generating a wide range of information for actionable insights and disseminating these for informed and effective decision-making to protect and improve the health of the population. ” The specific objectives of Public Health Surveillance will depend on what information is needed, who needs it and how it will be used (e.g: Monitoring & Evaluation vs. Emergency preparedness and response).
Early Warning and Response (EWAR)
Event-Based Surveillance (EBS) is defined as the organized collection, monitoring, assessment and interpretation of mainly unstructured ad hoc information regarding health events or risks, which may represent an acute risk to human health. The information collected for EBS is diverse in nature and originates from multiple, often not-predetermined sources both official and unofficial, including rumours reported by the media or ad hoc reports from informal networks. The information collection process is mainly active and carried out through a systematic framework specifically established for EBS purposes. ,
It offers key insights during various phases of outbreaks and emergencies, particularly in identifying such events and providing additional information during the response phase. As such, it's imperative to bolster EBS as an essential, integral part of national surveillance infrastructure. Especially in the context of regulatory intelligence following sources will play an important roles :
1) Internet-based EBS: Internet-based sources, both formal and informal, supply vital data for EBS. It is important that every country prioritizes strengthening this source of information. The World Health Organization's initiative, known as Epidemic Intelligence from Open Sources (EIOS), provides a useful platform for EBS, while promoting cooperative efforts through professional communities (see the Box).
2) Healthcare Worker EBS: Observations and reports by vigilant clinicians, lab staff, and other healthcare workers can often provide early warning signs. These could include an unusual cluster of symptoms, unique clinical presentations, or even diseases of unidentified origin. Therefore, it's necessary to establish and bolster a legal framework and procedures, and create an encouraging environment for such reporting.
Epidemic Intelligence from Open Sources (EIOS)
The Epidemic Intelligence from Open Sources (EIOS) initiative is a unique collaboration between various public health stakeholders around the globe. It brings together new and existing initiatives, networks and systems to create a unified all-hazards, One Health approach to early detection, verification, assessment and communication of public health threats using publicly available information. Since January 2022, the lead of the EIOS initiative is hosted within the new WHO Hub for Pandemic and Epidemic Intelligence. The EIOS Initiative has now over 60 Communities all over the world including 40 Member States. In the South-east Asia Region, the EIOS system has been implemented in four member states – Bangladesh, Indonesia, Nepal, and Thailand between 2022 and 2023. The regional office (SEARO) Health Emergency information management and Risk assessment (HIM) also uses the system on a daily bias to detect signals of potential public health importance in the region.
The EIOS system is a fit- for-purpose but constantly evolving web-based system designed to augment and accelerate global public health intelligence (PHI) activities, built on a long-standing collaboration between WHO and the Joint Research Centre (JRC) of the European Commission. It benefits from the experience gained in the Early Alerting and Reporting (EAR) project of the Global Health Security Initiative (GHSI) and the Hazard Detection and Risk Assessment System (HDRAS), as well as collaborations with other global initiatives and projects such as ProMED, the Global Public Health Intelligence Network (GPHIN), HealthMap and the Europe Media Monitor (EMM).
Each day, the system collates hundreds of thousands of articles from a broad range of sources, including traditional online media and specific social media sources, government and official web sites, news aggregators, blogs and expert groups, and collaborating initiatives. It runs these sources through a series of text mining and analytical modules to sort and categorize articles by topics, country, language, source and contextual indices. The system regularly checks for new information, which is downloaded and automatically processed and published through the secure EIOS user interface within a few minutes, accessible only to authorised individuals within the EIOS community. This leads to a continuous flow of new articles in the EIOS system, which registered users can interact with in various ways – both individually, as well as collaboratively with others. Within the system, each collaborating organisation has its own space and can securely share information within and across organisations.
EIOS technology does not replace the experts, but rather supports them, enabling them to rapidly sift through huge volumes of data from a wide variety of sources coming in at high velocity. This helps the identification of events in a fraction of the time it would otherwise take.
Sources of information for preparedness
To support regulatory intelligence as part of regulatory preparedness, SEARN pre-identified over 50 organizations and other sources of information covering the below needs identified by NRAs:
| Type of information | Examples | Sources of information |
|---|---|---|
| Regulatory guidelines |
|
|
| Clinical guidelines |
|
|
| Scientific information |
|
|
| Communication |
|
|
| Needs, access, supply, distribution |
|
|
| Safety information |
|
|
| Regulatory information |
|
|
Next steps
- Develop guidelines to NRAs to facilitate the implementation of the strategy.
- Support on any matter related to ensuring the preparedness of the Network.
References
- Infodemic [Internet]. [cited 2022 Nov 21]. Available from: https://www.who.int/health-topics/infodemic
- World Health Organization. Emergency response framework (ERF) [Internet]. 2nd ed. Geneva: World Health Organization; 2017 [cited 2023 Feb 7]. 68 p. Available from: https://apps.who.int/iris/handle/10665/258604
- Emergencies: International health regulations and emergency committees [Internet]. [cited 2023 Feb 7]. Available from: https://www.who.int/news-room/questions-and-answers/item/emergencies-international-health-regulations-and-emergency-committees
- World Health Organization, 2012. Rapid risk assessment of acute public health events. URL: https://www.who.int/publications/i/item/rapid-risk-assessment-of-acute-public-health-events
