Action Point 1 - Strategy to support capacity building
Last update: 04/07/2024
Background
One of the main objectives of SEARN is dedicated to ‘facilitate and support regulatory capacity development to enhance regulatory skills and competencies and strengthen regulatory systems in the region’. Capacity building was also one of the most common demands from the working groups during their meetings in January 2022 and from the Steering group in its March 2022 meeting.
To address this need, the Assembly created in 2022 a dedicated action point (AP1) in the SEARN workplan, led by Working Group 2 (WG2) Regulatory Strengthening.
Scope and definitions
While the concept of capacity building (later replaced by capacity development) has often been used synonymously with training and technical assistance, these are only some of several approaches to developing capacity (1).
The United Nations Sustainable Development Group (UNDG) defines capacity as ‘the ability of people, organizations and society as a whole to manage their affairs successfully’ and the United Nations Development Programme (UNDP) has defined capacity development as ‘the process through which individuals, organizations and societies obtain, strengthen and maintain the capabilities to set and achieve their own development objectives over time’ (2,3).
The UNDP identifies 3 inter-related levels to capacity development (2):
- Individual—improving individual skills, knowledge and performance through training, experiences, motivation and incentives;
- Organizational—improving organizational performance through strategies, plans, rules and regulations, partnerships, leadership, organizational politics and power structures, and strengthening organizational systems, processes, and roles and responsibilities
- Enabling environment—improving policy framework to address economic, political, environmental and social factors including economic growth, financing, labour markets, political context, policy and legislative environment, class structures, and cultural aspects in a coherent and mutually reinforcing fashion.
Specifically, the World Health Organization (WHO) has established a Regulatory System Strengthening (RSS) programme which uses the Global Benchmarking Tool (GBT) to identify strengths as well as areas for improvement, and develop Institutional Development Plan (IDP). In this context, this strategy generally encourages the Members of SEARN to use the GBT to strengthen their regulatory systems for all medical products. Of interest, the GBT defines competency as follows: ‘Competency combines knowledge, skills and attitude. Competencies describe how the work is to be carried out while objectives indicate what must be accomplished. They also provide a sound basis for consistent and objective performance standards by creating a shared language for what is needed and expected by the organization’.
This strategy focuses on:
- Individual capacity development
- Organizational capacity development, in complement to the WHO Regulatory System Strengthening (RSS) programme.
General objective
Capacity building to support Members in achieving their objectives of Maturity Level using the Global Benchmarking Tool and/or WLA status.
Principles guiding the development of the capacity building strategy
- The outcome of the capacity building strategy should benefit all Members of SEARN
- The strategy should rely on existing resources and only develop a new capacity building offer in areas where there are unmet needs
- The capacity building offer should be sustainable and predictable so NRAs can consider it in their training strategies
- The capacity building outcomes should be documented
Frameworks
UNDP levels for capacity development
Please see above (Scope and definitions)
Global Benchmarking Tool
The Global Benchmarking Tool (GBT) represents the primary means by which the World Health Organization (WHO) objectively evaluates regulatory systems, as mandated by WHA Resolution 67.20 on Regulatory System Strengthening for medical products (4). The tool and benchmarking methodology enables WHO and regulatory authorities to:
- identify strengths and areas for improvement;
- facilitate the formulation of an institutional development plan (IDP) to build upon strengths and address the identified gaps;
- prioritize IDP interventions; and
- monitor progress and achievements.
As part of this tool, all regulatory functions include sub-indicators related to the existence and implementation of guidelines, procedures, and tools, the development of which may be supported by collaboration and information sharing between the Members of SEARN.
Further, all regulatory functions include an indicator related to Human resources, containing several sub-indicators.
Global Competency Framework
The draft WHO Global Competency Framework for Regulators of Medical Products outlines competence criteria for pharmaceutical regulators and investigators. The global competency framework is designed to identify critical gaps in the professional development and capacity of regulatory personnel. The framework provides performance indicators across a variety of national regulatory functions, including the regulatory governance framework, marketing authorization/ reviewers, regulatory inspection, vigilance and surveillance, and laboratory analysis.
The competency framework’s provides means to evaluate NRAs development and a range of its capabilities to perform critical regulatory functions. Thus, the global competency framework allows competency modeling by individual NRAs across the maturity levels, in particular, levels 1 to 3, aligning individual capabilities with the organizational strategy and business processes.
The framework is organized around the competencies of an individual regulatory professional linked to the practice activities, which is used to define staff responsibilities. The defined practice activities are observable regulatory work inputs and outputs, integrating multiple competencies ,and the knowledge, skills, and behaviors for that activity. Practice activities are either core activities that are linked to the organizational regulatory system as defined in the WHO GBT or role-specific practice activities for reviewers, pharmacovigilance, laboratory analysts, and inspectors.
Prioritized needs
As per the strategy adopted in July 2023, the Network decided to prioritize piloting the development of the preferred domains from each dimension of the draft Global competency framework based on the results of the survey:
- Meta-Competencies (Collaboration),
- Core-Competencies (regulatory framework, policies, and process),
- Role-specific competencies for reviewers (Product quality),
- Inspectors (Regulatory decision-making),
- Laboratory analysts (Laboratory systems and equipment)
- Vigilance personnel (Post-market surveillance).
Piloting the development of a sustainable capacity building
Vision
A vision was developed for the capacity building mechanism in SEARN. It is summarized and further described below:
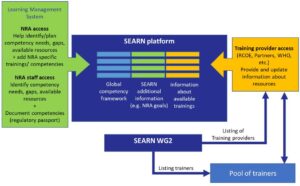
The following key components are foreseen:
- At the center of the system is a platform, based on the Global Competency Framework, complemented by additional information (e.g. NRA goals), which together form the SEARN competency framework.
- NRAs may use this platform or an associated system as a learning management system to help them identify their competency needs, resources and gaps based on their goals, and plan/budget their trainings accordingly
- NRA staff may use this platform to identify their individual competency needs and gaps based on their personal goals and the goals of their NRA, access resources, and document their competencies in their regulatory passport.
- Training providers, including regional centers of excellence, partners, WHO and others, may use this platform to provide an update information about their resources against the SEARN competency framework.
- SEARN WG2 is responsible of listing and reviewing training providers, and would also constitute a pool of trainers which can be used as resources by training providers.
SEARN capacity building platform
The competencies and activities from the prioritized domains were reformatted in an excel spreadsheet, which further identified key information which would characterize activities and training programmes proposed by training providers to address these needs.
Activities vs. competencies
Consensus was built on how to best utilize the components of the Global competency framework to support this strategy:
- Activities should be the entry point for NRAs. These will help them answer the question: which activities should staff perform to achieve the NRA’s mission and goals.
- Competencies*, are helpful to training providers for either supporting them in developing their training through identifying which competencies are required to perform an activity, or to inform NRAs about the content of their proposed trainings. It is likely that a training programme designed to enable staff to perform one activity will require multiple core-, meta- and specific competencies.
*As per the Global competency framework, competencies are the knowledge, skills, attitudes and behaviours needed by people within an organization, which are developed through education, training and experience. Competencies can be measured through the performance of tasks.
NRA goals
For the purpose of this strategy, the SEARN Members agreed on 3 NRA goals for medicines and vaccines. For each NRA goal, a basic algorithm was developed to link activities and goals as follows:
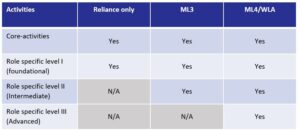
This basic algorithm was complemented by a review from the secretariat and a written consultation to WG1, WG2 and WG3 to ensure there was agreement on the classification of the activities according to the NRA goal.
Learning management system and regional regulatory passport
Consensus was build to use the platform or some linked system as a learning management system. This LMS would also assist in documenting the competency of the NRA’s staff. The system would build on the concept of regulatory passport, which was conceptualised for the benefit of this strategy by the Northeastern University (please refer to Annex 2).
The regulatory passport was defined as a ‘digital documentation, designed to record an individual's (mandatory) training activities and competencies’. It relies on five key features:
- Comprehensive Record-Keeping
- Regional/global recognition
- Mitigation of redundancy
- Facilitation of Mobility
- Continuous professional development
Training providers and catalogue
Characteristics of training programmes and guidelines to develop new resources
Additional work was conducted to identify what would be the key information to capture to characterize a training programme.
A table was developed which can be used to guide training providers in describing their training programmes to NRAs, and it was completed with some guidance to assist training providers in developing new resources to enable NRA staff to perform an activity.
Pilot of the mapping of existing resources against the global competency framework
Partners were requested to map their existing resources against the global competency framework to ensure the feasibility, address arising issues, and initiate the development of a catalogue of resources in the SEARN platform.
Role and function of WG2
Consensus was built on the possibility to expend the role of WG2, possibility assisted with external experts, to perform two new activities supporting this strategy:
- Listing training providers
- Listing trainers which could be used as a resource by training providers to implement their training programmes.
To enable these new activities, some changes have proposed in the terms of reference of the Network.
Regional Centres of Regulatory Excellence (RCOE)
A survey was conducted to identify potential regional centers of regulatory excellence (RCOEs), which was identified as a core component for this strategy. An RCOE could be either an NRA or and academic institutions.
Highlights:
- Potential RCOEs, either NRA or from other institutions have been identified in all selected domains
- While several institutions were identified for pharmacovigilance and laboratories, only one institution was identified at this stage for Marketing Authorizations for Pharmaceuticals and Regulatory Quality Management Systems
- Limited academic institutions were identified, suggesting further outreach efforts could be made.
Next steps
- Continue the development of a sustainable capacity building offer in the prioritised domains of competency in relation with the countries’ tailored goals, including RCOEs
- Support the development of a capacity building platform for SEARN
- Support the finalisation and implementation of the expansion of the composition of WG2 (as needed) to support listing of training providers and a pool of trainers
- Pilot the development and implementation of a regional programme of GSDP workshops
- Organize a webinar to disseminate the SEARN strategy for capacity building, including to HR departments and universities/relevant training providers
- Develop a domain in the competency framework to support NRAs in ensuring safe medicines during pregnancy
References
- Bester A. Capacity Development: A Report Prepared for the United Nations Department of Economic and Social Affairs for the 2016 Quadrennial Comprehensive Policy Review [Internet]. 2015. Available from: https://www.un.org/en/ecosoc/qcpr/pdf/sgr2016-deskreview-capdev.pdf
- UNDP. Capacity development - UNDAF Companion Guidance [Internet]. 2017. Available from: https://unsdg.un.org/resources/capacity-development-undaf-companion-guidance
- UNDP. UNDP and capacity development | United Nations Development Programme | about [Internet]. [cited 2022 Oct 23]. Available from: https://www.undp-capacitydevelopment-health.org//en/about-us/capacity-development/
- WHO. Global Benchmarking Tools [Internet]. [cited 2022 Dec 8]. Available from: https://www.who.int/tools/global-benchmarking-tools
- About WHO: Collaborating centres [Internet]. [cited 2022 Dec 8]. Available from: https://www.who.int/about/collaboration/collaborating-centres
