Action Point 16 - Strategy to develop a regional mechanism to detect and review safety signals
Last update: 04/07/2024
Background
As highlighted in the SEARN strategy to stimulate reporting in the region which was adopted on 27 July 2023, the level of reporting in the region is generally lower than what is observed in other parts of the world. While a strategy has been updated to address this critical gap, increasing reporting will likely take time. Meanwhile, National Regulatory Authorities (NRAs) are required to ensure the safety of the products on their market.
While reliance can be advantageously used to monitor the safety of medical products and keep the product information updated, this sole approach presents two main limitations:
- Specificities in the region, whether related to biological reasons or to the organisation and functioning of the societies, may not be detected in other parts of the world
- Some medical products do not exist in countries with an adequate reference NRA, or may only be exceptionally used in other parts of the world.
Further, there may be further opportunities for regional collaboration in the assessment of safety signals raised by the Member States, to support their regulatory decisions. Finally, optimizing the use of the reports and communicating on how reports are used to improve the safety of patients was identified as one of the means to encourage patients and healthcare professionals to report.
Considering these challenges, the Assembly created in 2023 a dedicated action point (AP16) in the SEARN workplan, led by Working Group 3 (WG3) Vigilance.
Main objective
To optimize the use of the vigilance reports collected.
Definitions and scope
The scope of the strategy was limited to medicines and vaccines at this stage.
According to the report from the CIOMS Working Group VIII Practical Aspects of Signal Detection in Pharmacovigilance, a signal is defined as “information that arises from one or multiple sources which suggests a potentially new causal association, or a new aspect of a known association, between an intervention and an event or set of related events, either adverse or beneficial, that is judged to be of sufficient likelihood to justify verificatory action.” WGVIII Report (cioms.ch)
A more recent clarification of the definition provided by the UMC is that a signal “is essentially only a hypothesis [of a causal association between an invention and an event] that, together with data and arguments, justifies the need for further assessment.” What is a signal? | UMC (who-umc.org)
For the purpose of this strategy, it was agreed to use the definition from UMC, also quoted by the CIOMS Cumulative Glossary with a focus on pharmacovigilance as:
Information on a new or known side effect that may be caused by a medicine and is typically generated from more than a single report of a suspected side effect. It is important to note that a signal does not indicate a direct causal relationship between a side effect and a medicine, but is essentially only a hypothesis that, together with data and arguments, justifies the need for further assessment.
Source: Uppsala Monitoring Centre (UMC). What is a signal?
Regarding the criteria of novelty, it was agreed that a signal would be considered as new if the information is not already mentioned in the product information of SEARN members and some reference NRAs for pharmacovigilance, to be further identified.
Assessment steps
The most complete and transparent guidance in signal management has been provided by the EMA in Good Pharmacovigilance Practices IX. A generally applicable interpretation of the steps of signal management includes the following activities: signal detection, signal validation, signal prioritisation, signal assessment and recommendation for action.
Signal detection
Signal detection is the process of looking for and/or identifying signals using data from any source. Signal detection in passive reporting databases can be done by qualitative (“case-by-case") or quantitative (statistical screening by disproportionality analysis) approaches.
Signal validation
Signal validation is the process of evaluating the data supporting the detected signal in order to verify that the available documentation contains sufficient evidence and therefore justifies further analysis of the signal. Signal validation should take into account the strength of the evidence, the clinical relevance and the previous awareness of the association. It can be thought of as a form of preliminary assessment as the extent of evaluation performed during signal validation versus further assessment can vary between individuals/institutions.
The following elements should be considered when performing signal validation based on the review of ICSR data:
- Previous awareness, e.g.:
- the extent to which information on the adverse reaction is already included in the product information (summary of product characteristics (SmPC) and package leaflet);
- whether the signal relates to an adverse reaction already included in the SmPC for other medicinal products containing the active substance of interest, bearing in mind that some signals may only be relevant to a specific medicinal product and/or a specific formulation
- whether the association has already been assessed in the initial application for marketing authorisation, the risk management plan (RMP), the periodic safety update report (PSUR) or any other regulatory procedure, based on information held or known by each organisation;
- Strength of the evidence, taking into account, e.g.:
- the total number of cases (after exclusion of duplicates), and amongst those, the number of supportive cases, e.g. cases showing a compatible temporal association, positive de- or rechallenge, lack of potential alternative causes, assessed as possibly related by the reporting healthcare professional, with supportive results of relevant investigations;
- number of cases in the context of patient exposure;
- additional cases reported with related terms (e.g. other MedDRA terms indicating clinical complications or different stages of the same reaction);
- consistency of the evidence across cases (e.g. consistent time to onset, pattern with repeated observations of an association); − quality of the data and their documentation;
- cases matching internationally agreed case definitions if applicable (e.g. Brighton collaboration case definitions for vaccines, RegiSCAR criteria for severe cutaneous adverse reactions);
- dose-reaction relationship;
- possible mechanism based on a biological and pharmacological plausibility;
- disproportionality of reporting, if applicable (see GVP Module IX Add I).
- Clinical relevance and context, e.g.:
- seriousness and severity of the reaction;
- outcome and reversibility of the reaction;
- additional insight on a known adverse reaction, e.g. in terms of its severity, duration, outcome, incidence or management;
- reactions occurring in the context of drug-drug interactions;
- reactions occurring in vulnerable populations, children or the older populations, or in patients with pre-existing risk factors;
- reactions occurring in different patterns of use (e.g. overdose, abuse, misuse, off-label use, medication errors, falsified products);
- Additional sources of information may provide further evidence for or against a causal association, or a new aspect of a known association, and may be considered during further assessment of the signal, depending on their relevance for the signal and availability to each organisation. These may include:
- clinical trial data;
- findings regarding similar cases in the scientific literature, including information on substances of the same class of medicinal products;
- information on the epidemiology of the adverse reaction or the underlying disease; • experimental and/or non-clinical findings;
- databases with larger datasets, when the signal was detected from national or marketing authorisation holder-specific databases;
- healthcare databases that may provide information on characteristics of exposed patients and medicines utilisation patterns;
- information from other regulatory authorities worldwide
Signal prioritisation
Signal prioritisation is the process in which is determined whether a validated signal requires further assessment. This is based on an initial analysis of the potential impact of the signal on patients’ or public health and the risk-benefit balance of the concerned medicinal product(s).
Institutions which can provide support in the assessment of safety signals
Indian Pharmacopoeia Commission
The IPC Collaborating center agreed to provide technical support to this strategy, as well as training and information sharing (e.g. SOPs). In addition, the organisation agreed to support signal detection at regional level and dissemination of information.
Uppsala Monitoring Centre
The UMC agreed to provide technical support in the development of this strategy.
Monitoring and detecting safety signals at the regional level
Challenges
Three main challenges have been identified in monitoring, detecting, and assessing safety signals at the regional level:
- The legal possibility to share case narratives
- The technical possibility to access case narratives at the regional level
- Insufficient capacity in some countries.
In order to share confidential information, such as case narratives, some countries may need on the short term to sign MoU or confidential disclosure agreement with the IPC.
Monitoring and detecting safety signals at the regional level
The monitoring and detection system will be based on the work from the IPC Collaborating Centre, which will identify safety signals for priority products in VigiBase at the regional level:
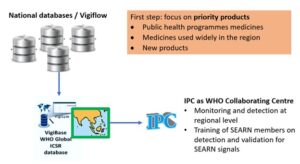
Priorities
As a first step, it was agreed to prioritize regional collaboration on medicines from the following category:
First step: Priority medicines:
- Public health programmes medicines
- Medicines used widely in the region
- New products
Among these categories, the main objectives of this surveillance programme would be to identify:
- Particularities due to populations/public health systems in the region, including for molecules which are already well known in other parts of the world
- New adverse drug events/new aspects in ADE for new medicines
- Substandard/falsified products with adverse consequences (e.g. due to microbiological or chemical contamination)
On this basis, a list of priority medicines was adopted. In addition, the Assembly recommended to include pregnancy adverse events in the priority list for monitoring /detection at the regional level.
List of priority medicines:
| Category | Active Substance(s) |
|---|---|
| Public health programmes medicines | Dapsone |
| Public health programmes medicines | Kanamycin |
| Public health programmes medicines | Isoniazid |
| Public health programmes medicines | Ethionamide |
| Public health programmes medicines | Ethambutol |
| Public health programmes medicines | Efavirenz |
| Public health programmes medicines | Doxycycline |
| Public health programmes medicines | Dolutegravir |
| Public health programmes medicines | Diethylcarbamazine |
| Public health programmes medicines | Darunavir |
| Public health programmes medicines | Lamivudine |
| Public health programmes medicines | Clofazimine |
| Public health programmes medicines | Clarithromycin |
| Public health programmes medicines | Chloroquine |
| Public health programmes medicines | Capreomycin |
| Public health programmes medicines | Bedaquiline |
| Public health programmes medicines | Atazanavir |
| Public health programmes medicines | Artesunate |
| Public health programmes medicines | Artemether |
| Public health programmes medicines | Nevirapine |
| Public health programmes medicines | Zidovudine |
| Public health programmes medicines | Rifampicin |
| Public health programmes medicines | Raltegravir |
| Public health programmes medicines | Quinine |
| Public health programmes medicines | Pyridoxine |
| Public health programmes medicines | Pyrazinamide |
| Public health programmes medicines | Primaquine |
| Public health programmes medicines | Paramomycin |
| Public health programmes medicines | Amphotericin B deoxycholate |
| Public health programmes medicines | Moxifloxacin |
| Public health programmes medicines | Miltefosine |
| Public health programmes medicines | Mefloquine |
| Public health programmes medicines | Lumefantrine |
| Public health programmes medicines | Lopinavir |
| Public health programmes medicines | Liposomal Amphotericin B |
| Public health programmes medicines | Linezolid |
| Public health programmes medicines | Levofloxacin |
| Medicines used widely in the region | Cefradine |
| New products | Fesoteroterodine |
| New products | Dalbavancin Hydrochloride |
| New products | Crisaborole |
| New products | Cannabidiol |
| New products | Asciminib |
| New products | Abrocitinib |
| Medicines used widely in the region | Mefenamic Acid |
| Medicines used widely in the region | Imatinib |
| New products | Imeglimin Hydrochloride |
| Medicines used widely in the region | Loperamide |
| Medicines used widely in the region | Dextromethorphan |
| Medicines used widely in the region | Ibuprofen + Paracetamol |
| Medicines used widely in the region | Olanzapine |
| Medicines used widely in the region | Phenylephrine |
| Medicines used widely in the region | Tetracycline |
| Medicines used widely in the region | Amoxicillin |
| Medicines used widely in the region | Paracetamol |
| New products | Prussian Blue Insoluble +/- Magnesium Hydroxide |
| Public health programmes medicines | Albendazole |
| Public health programmes medicines | Abacavir |
| New products | Trifarotene |
| New products | Treprostinil |
| New products | Tirzepatide |
| New products | Sovateltide |
| New products | Remifentanil Hydrochloride |
| New products | Relugolix |
| Medicines used widely in the region | NSAIDs |
| New products | Polmacoxib |
| New products | Plecanatide |
| New products | Plazomicin |
| New products | Niraparib |
| New products | Lobeglitazone Sulfate +Glimepiride |
| New products | Lifitegrast |
| New products | Lasmiditan |
| New products | Inclisiran |
Responsibilities
SEARN Members
SEARN Members should collaborate with the IPC collaborating centre to support the validation and assessment of signals detected at the regional level.
IPC Collaborating Centre
The IPC collaborating centre will be responsible to:
-
- monitor and detect signals at the regional level using VigiBase
- train SEARN members on the detection and validation of SEARN signals
Signal detection
The process of identifying potential signals pertaining to Adverse Drug Reactions (ADRs) is dependent on spontaneous ADR reporting from across the region. SEARN Members and IPC review Individual Case Study Reports (ICSRs) submitted through the VigiFlow Database, maintained by the World Health Organization (WHO).
Any unusual or unknown ADR, or statistical significance identified by VigiLyze, is flagged for further review after determining the accuracy of the available information from the ICSRs.
Signal validation
The IPC conduct a preliminary assessment to determine if the available information contains sufficient evidence to justify further analysis of the signal, including:
- Similar cases, if any, are also collected.
- data sources such as published articles, drug pamphlets provided by the manufacturers etc.,
- more information (e.g. case narratives) provided by SEARN members, as needed.
- product information of SEARN members and some reference NRAs for pharmacovigilance (to be further agreed upon) to verify if the ADR reported is already listed or if the characteristics are as described.
If the signal is validated, the preliminary assessment is shared with SEARN WG3 for further review.
Assessment of national safety signals at the regional level
The assessment system will be based on recommendations provided by a regional committee composed of additional experts, based on safety signals detected either by the regional monitoring system or through other sources (such as signals detected/validated by SEARN members or the secretariat):
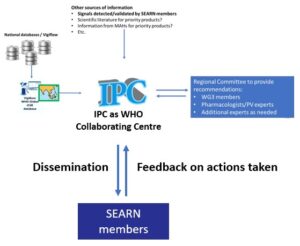
Objectives
To detect and assess safety signals at the regional level for priority products and on the request of one SEARN member or the SEARN secretariat, and advise SEARN Members on:
- The possibility of a causal relationship based on all available evidence,
- the need for risk minimization measures including regulatory actions (e.g. package insert and label etc.),
- the need for further studies to address remaining uncertainties.
Detection and validation at the national level
NRAs and national pharmacovigilance centres are responsible for detecting and validating safety signals at the national level. SEARN will support this work through the identification or development of training, guidelines and templates.
Prioritization
The IPC will review the preliminary assessment report from the SEARN member or secretariat to determine whether the signal requires further assessment, considering the potential impact of the on patients’ or public health and the risk-benefit balance of the concerned medicinal product(s).
If a signal was not prioritised, the IPC will initiate a discussion with the SEARN member or secretariat. If a divergence persisted, the issue will be brought to WG3 to decide the next steps.
Assessment
The organization who raises a signal is responsible for its assessment prior the regional discussions. Support may be arranged from Secretariat or another SEARN Member when the need arises. SEARN will support this work through the identification and development of training, guidelines and templates.
The composition of WG3 will be expended to include relevant experts to support this work. WG3 will propose some dedicated terms of reference to be approved by the Steering group.
The secretariat will develop a system to ensure adequate identification and management of the links of interest of external experts.
Recommendations, dissemination and feedback
The recommendations will be published on the website of SEARN and shared with members of WG3 and focal points.
NRAs will also be requested to provide feedback on how these recommendations were considered through the Monitoring and Evaluation framework.
The IPC will initiate a regional newsletter to facilitate information sharing between SEARN members. SEARN members should share with the IPC Collaborating Centre information about their activities, successes, challenges, etc. This can also be achieved through sharing the national pharmacovigilance newsletters
Mapping of ADR reporting forms against the core variables identified by WHO
A mapping exercise was conducted of national ADR reporting forms in SEARN against the core variables identified by WHO.
The final results will be shared in a scientific publication.
Recommendations and next steps
Recommendations
SEARN requested:
- WHO and UMC to explore means to share information (e.g. case narratives) at the regional level.
- SEARN members to consider the WHO core variables for reporting suspected adverse drug event reports and review their national ADR reporting forms as needed.
- SEARN members to share with the IPC Collaborating Centre information about their activities, successes, challenges, etc., which can also be done through sharing their national pharmacovigilance newsletters to provide content for the regional newsletter.
Next steps
- Initiate the regional monitoring of priority medicines by 1st August 2024
- Explore the expansion of priority medicines to vaccines and biologicals
- Support the finalisation and implementation of the expansion of the composition of WG3 to support signal assessment (including the dedicated terms of reference, the nomination of additional experts, and the creation of an experts database)
- Identify or develop trainings, guidelines and templates to support signal validation and assessment
- Deepen the description of the variables in the national ADR reporting forms’ and publish the results in a scientific paper
- Organize an in-person workshop with key stakeholders for in-depth discussions on how to strengthen pharmacovigilance in the region
