Action Point 5 - Strategy to facilitate reliance
Last update: 04/07/2024
Background
The South-East Asia Regulatory Network (SEARN) was created in 2016 by the 11 Member States of the WHO South-East Asia Region to develop and strengthen regulatory collaboration, convergence and reliance in the South-East Asia region over shared regulatory issues and challenges.
As a matter of fact, one of the main objectives of the network is to ‘Identify and develop potential work sharing and reliance processes to help address common work areas and optimize use of existing regulatory capacities and expertise available in the region.’
To address this need, the Assembly created in 2022 a dedicated action point (AP5) in the SEARN workplan, led by Working Group 2 (WG2) Regulatory Strengthening.
Scope and definitions
In 2021, the WHO Expert Committee on Specifications for Pharmaceutical Preparations adopted as an annex to its 55th report the Good reliance practices in the regulation of medical products: high level principles and considerations.
This document defines reliance as ‘The act whereby the regulatory authority in one jurisdiction takes into account and gives significant weight to assessments performed by another regulatory authority or trusted institution, or to any other authoritative information, in reaching its own decision. The relying authority remains independent, responsible and accountable for the decisions taken, even when it relies on the decisions, assessments and information of others’.
As Figure 1 describes, reliance may take many forms and be applied to varying degrees in recognizing or taking account of the assessments, decisions or other authoritative information of other authorities and institutions. While recognition may be seen as a special and more formalized approach to reliance, whereby one regulatory authority recognizes the decisions of another regulatory authority, system or institution, obviating additional regulatory assessment to reach its own decision. Recognition usually requires formal and binding legal provisions.
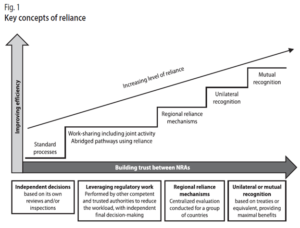
Reliance may be used in the regulation of any of the medical products in the scope of SEARN and for all regulatory functions, in the full life cycle of a medical product.
While the precise situations in which reliance may be used are to be defined at the national level, some conditions are required to enable reliance, including having access to sufficient information from the reference NRA such as full/public assessment reports in a common language documenting their regulatory decisions.
General objective
To facilitate reliance in the SEARN region.
Enabling reliance in the region
Prioritized areas for reliance
The prioritized areas were selected considering the results from a survey conducted during the 2022-2023 work plan. It was decided that this strategy would focus on:
- medicines and vaccines,
- For Marketing Authorizations, Vigilance, Regulatory Inspections, and Laboratory Testing.
Converging on the definition of reference regulatory authorities in SEARN
The WHO Good reliance practices in the regulation of medical products: high level principles and considerations define a reference regulatory authority as a ‘national or regional authority or a trusted institution such as WHO prequalification (WHO PQ) whose regulatory decisions and/or regulatory work products are relied upon by another regulatory authority to inform its own regulatory decisions’.
The guidelines further highlight that ‘Each NRA should define its own strategy for an appropriate risk-based approach to reliance, which includes factors such as the type and source of products evaluated, the level of resources and expertise available in the NRA, the public health needs and priorities of the country and opportunities for reliance’.
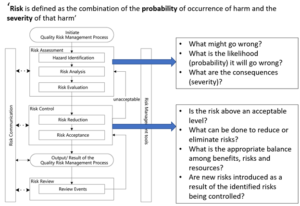
Further information on Quality Risk Management can be found in ICH Q9(R1) – 2021 and in the 2013 WHO guidelines on Quality Risk Management. The approach is summarized in the below diagram derived from ICH Q9(R1):
When identifying a reference regulatory authority, the below principles can be followed:
- It should be based on objective criteria, including evidence that the authority can be trusted (e.g. WLA, benchmarking, audit, accreditation, other information about the actions taken by an NRA), and the possibility to access the minimum required information in a language that is understood or can be easily translated.
- It should consider the capacity of the reference NRA to conduct the regulatory function independently (e.g. maturity level, or technical capacity to review further a dossier) and the capacity of the relying NRA compared to the reference regulatory authority to conduct the activities of a certain function (i.e. can the reference NRA do the assessment as well or better than the relying NRA? However, a minimum level of ML3/4 or WLA should be expected)
- It should be specific to one particular type of products and regulatory function (e.g. an authority may be ML3 for vaccines but not for medicines, or ML4 for vigilance but ML1 for marketing authorizations)
- It should be reconsidered regularly based on experience (e.g. for Marketing Authorizations, considering the frequency of substandard and falsified products).
- As a best practice, the relying NRA when applying reliance, should at least verify sameness. A checklist and guidance to conduct verification of product sameness for NRAs to use as reference can be found in the Appendix 2 of the 53rd report of the WHO Expert Committee on Specifications for Pharmaceutical Preparations (page 259) for Marketing Authorizations and Regulatory Inspections)
- It is recommended to publish on the NRA website a guideline on reliance, which defines the scope, criteria and principles followed to select reference authorities and how such assessment will be used, together with information which may be updated more regularly on which are the accepted reference regulatory authorities (for one particular type of product/function or type of decision). This will help stakeholders to comply with the NRA’s expectations.
Possible Criterion for selecting a Reference Regulatory Authority
In the context of the prioritized functions, the table below presents some examples of criteria which may be considered to define the relying strategy:
| Regulatory function | Possible Criterion for selecting a Reference Regulatory Authority |
|---|---|
| Marketing Authorizations |
|
| Vigilance |
|
| Regulatory Inspections |
|
| Laboratory Testing |
|
Useful links
- List of WHO Listed Authorities WLAs: https://www.who.int/publications/m/item/list-of-who-listed-authorities-wlas
- List of transitional WLAs: https://www.who.int/publications/m/item/list-of-transitional-wlas
- Definition of a Stringent Regulatory Authority: https://www.who.int/publications/i/item/9789241210034
- List of WHO prequalified quality control laboratories: https://extranet.who.int/pqweb/medicines/prequalified-lists/sf-quality-control-labs
Types of decisions for which reliance may be used within the prioritized functions and sources of information
For each prioritized areas, a diversity of decisions for which reliance may be used was identified. Beyond, reliance can also be used for capacity development (e.g. when developing a new guideline or a new SOP, one Member may use the document from another country as a reference or as a starting basis).
For Marketing authorizations for medicines and vaccines, reliance may be used for:
- Initial marketing authorizations, including product information
- Renewal of marketing authorizations
- Variations, including e.g. new indications or changes in the posology
- Suspension, withdrawal of a Marketing Authorization
For Vigilance for medicines and vaccines, reliance may be used for:
- Safety signal detection and assessment
- Safety variations, including changes in the SmPC and patient information leaflet
- PSUR assessment
- Risk communication (e.g. Dear doctors letters, Direct Healthcare professional communications)
- Risk management plans
- Benefit-Risk reviews
For Regulatory inspections for medicines and vaccines, reliance may be used for:
- GxP compliance, including initial assessment and maintenance
- Information for risk-based planning of inspections, e.g. history of recalls
For Laboratory testing for medicines and vaccines, reliance may be used for:
- Quality test results (prior or after registration/marketing)
For Market control and surveillance: while information sharing is critical for market control and surveillance, no regulatory decision could be identified for which reliance would be used, as this activity is national in nature. Associated decisions for which reliance may be used, such as benefit-risk reviews or testing, have already been addressed.
Overall, initial discussions highlighted that for the above decisions, possible sources of information, could include:
- Applicants
- Other NRA websites and WHO website (e.g. for prequalified products or guidelines)
- Documents directly provided by other NRAs (e.g. un-redacted assessment report)
- The collaborative registration procedure (CRP)
Minimum information required for reliance
The below document was developed to support the implementation of this strategy. There would be 2 main usages of this information:
- Recommendations to guide relying countries on what is required as a minimum for them to be able to rely on other organizations.
- Recommendations to SEARN countries on what to publish/make available (in relation with AP3 information sharing and AP4 internal platform) in order to facilitate reliance from other countries on their own decisions.
Acknowledging that the implementation of these recommendations may require addressing practical and technical challenges, and in some cases further discussions and agreement of other authorities, the adoption by the Assembly of SEARN will be followed by an implementation period of two years.
The information identified in the below table intends to present the minimum information required for reliance. For reliance, the ultimate requirement is that the relying NRA should have sufficient trust in the reference authority to use the output of their work in their own regulatory decision-making system. Abridged assessment may require additional information.
Reference organisations currently used in the region
A survey was conducted to identify the reference organizations currently used in the region. The scope was medicines and vaccines only. The survey was divided between reference organizations for Marketing Authorisation/Regulatory Inspections and Laboratory testing.
Highlights:
- Legacy remains an important driver regarding the choice of reference regulatory authorities, and the status of Stringent Regulatory Authorities (SRA) remains the main factor associated with reliance in the region
- Some inconsistencies have been observed. For example only one Member indicated relying on Saudi Arabia while its regulatory system reached Maturity Level 4 for both medicines and vaccines. Another example was that despite its SRA status, not all relying Members identified Health Canada.
- Some other uncertainty or inconsistencies were observed. For regulatory inspection, only some PIC/S members are relied upon, not all.
- Overall, the intra-regional reliance is very low.
- A lower level of reliance was reported for Laboratory testing, which could either reflect less reliance or the profile of those who responded to the survey. Similar inconsistencies and low intra-regional reliance was observed.
Mapping of the sources of information for reliance
Reference organisations for SEARN
Based on the results from the survey and further discussion, it was agreed for the purpose of the mapping of the sources of information for reliance prioritize reference regulatory authorities as follows:
- EMA (pilot)
- TGA
- US FDA
- MHRA
- Japan
- SwissMedic
Piloting of the mapping of the sources of information for reliance
It was agreed to initiate the piloting of the mapping of the sources of information with EMA. The results are provided below.
If you identify any error or broken link, please report it to the SEARN secretariat by email or using the contact form.
| Regulatory function | Type of decision | Reference organization | Source of information | Comments | |
|---|---|---|---|---|---|
| Marketing authorizations | Initial marketing authorisations | EMA | Database of authorised medicines: https://www.ema.europa.eu/en/medicines
General Product page: https://www.ema.europa.eu/en/medicines/human/EPAR/pandemic-influenza-vaccine-h5n1-astrazeneca-previously-pandemic-influenza-vaccine-h5n1-medimmune Product EPAR: https://www.ema.europa.eu/en/documents/overview/pandemic-influenza-vaccine-h5n1-astrazeneca-epar-summary-public_en.pdf |
| |
| Marketing authorizations | Product information | EMA | Search product in: https://www.ema.europa.eu/en/medicines
General Product page: https://www.ema.europa.eu/en/medicines/human/EPAR/pandemic-influenza-vaccine-h5n1-astrazeneca-previously-pandemic-influenza-vaccine-h5n1-medimmune |
| |
| Marketing authorizations | Renewal of marketing authorisations | EMA | Search product in: https://www.ema.europa.eu/en/medicines |
| |
| Marketing authorizations | Variations | EMA | Search product in: https://www.ema.europa.eu/en/medicines |
| |
| Marketing authorizations | Suspension and withdrawal of marketing authorisations | EMA |
|
| |
| Vigilance | Safety signals | EMA |
| ||
| Vigilance | Safety variations | EMA | Please refer to variations | ||
| Vigilance | PBRER | EMA |
|
| |
| Vigilance | Risk communication | EMA |
|
| |
| Vigilance | Risk management plans | EMA |
|
| |
| Vigilance | Benefit-risk reviews | EMA |
| ||
| Regulatory inspections | GMP compliance | EMA |
|
| |
| Regulatory inspections | GCP Compliance | EMA | General information on GCP inspections:
|
| |
| Regulatory inspections | GSDP compliance | EMA |
|
| |
| Regulatory inspections | Information for risk-based planning of inspections | EMA |
|
| |
| Laboratory testing | Quality test results | EMA | Not applicable |
Next steps
- Develop a strategy for reliance on marketing authorization of priority products
- Continue the mapping of reference NRAs
- Explore how to further consider WLAs in this strategy
- Propose means to address any issue arising in the scope of this strategy
