Action Point 10 - Strategy to stimulate reporting
Last update: 27/07/2023
Background
Regulators, program managers, manufacturers, and eventually Healthcare professionals and patients rely on different sources of information to keep their knowledge of a product up to date and ensure its use is safe and effective. Sources of information include scientific literature, clinical trials, observational studies, information published by other regulators and institutions, and spontaneous reporting of events.
Spontaneous reporting remains a key step to several regulatory activities which are key to ensuring the quality, safety and effectiveness/performance of medical products. These regulatory activities include vigilance, market surveillance (by NRAs for medical devices), post introduction surveillance by immunization programs, post-market surveillance (by manufacturers of medical devices), and market control (for substandard and falsified products).
The Council for International Organizations of Medical Sciences defines a spontaneous report as ‘an unsolicited communication by healthcare professionals or consumers to a company, regulatory authority or other organization that describes one or more suspected adverse drug reactions in a patient who was given one or more medicinal products’ (CIOMS 2021). However, spontaneous reporting concerns other situations which share the characteristic of being associated to a demonstrated or potential risk for patients or users, including adverse drug events (ADEs), adverse events following immunization (AEFI), feedback from medical devices users, quality defects, medication errors, etc. Further, WHO generally distinguishes the notification, when the health system (e.g. a healthcare professional) is informed about an event, and reporting, when the event is communicated (usually in pre-defined formats) to regulators, program managers, and manufacturers through paths defined at the national level for further analysis and, as required, to decide on risk minimisation measures (WHO n.d.).
While reporting contributes to building shared international knowledge which is used by the relevant actors, national reporting is also important to detect country-specific risks (e.g. products only marketed in the region, particular drug-drug interactions, regional specificities, programme errors etc.), quality issues, and to provide transparent and authoritative information to the public.
In general, and depending on national regulation and guidelines (e.g. program implementation guidelines), reports can be issued by different users, including healthcare professionals (e.g. medical doctors, pharmacists, midwives, nurses, etc.) patients, their caregivers, and manufacturers. These reports, once notified, are collected by manufacturers, program managers, and regulatory authorities to detect, analyse and minimize risks.
While there is not always a gold standard defining what level of reporting is adequate, it has generally been observed that the level of reporting in SEARN countries would benefit from being further encouraged. Some countries only exceptionally receive reports, while other collect more reports but once compared to the population the rate often remains lower than what is observed in other parts of the world. Finally, there is also diversity in the level of reporting between the different products, and lessons may be learned from these different experiences.
Considering that low-reporting in the region, while complex, may prevent or delay the detection of serious risks, or hamper adequate analysis and risk minimisation measures, the Assembly of SEARN adopted on 8 June 2022 the SEARN Work Plan 2022-2023 which includes the action point 10, led by Working Group 3 (WG3) Vigilance: ‘Draft a strategy to stimulate reporting in the region, including technical solutions’. This work shall be embedded in the scope and objectives of WG3, which include all medical products that are in the scope of SEARN and also consider substandard and falsified products.
While there are specificities to the different types of reports, it is expected that this strategy may address many of the common challenges and solutions.
Objectives
To identify challenges to notification of events to the health system and other entities (e.g. marketing authorization holders, manufacturers etc) and its spontaneous reporting and practical solutions which may be used by countries and WHO in the region to take adequate and timely action to minimise the risks.
Identification of the main barriers reported in the literature
Method
To identify the main barriers to reporting in the region:
- reviews on barriers to reporting were identified in the literature
- this list of publications was further completed with requests to SEARN members for any other relevant publications
- the identified barriers were identified in the publications and categorized
- two experts from Bangladesh and India reviewed the list of identified barriers to ensure relevance and comprehensiveness
- the lists were further reviewed in the relevant working groups of SEARN
Main barriers identified in the region
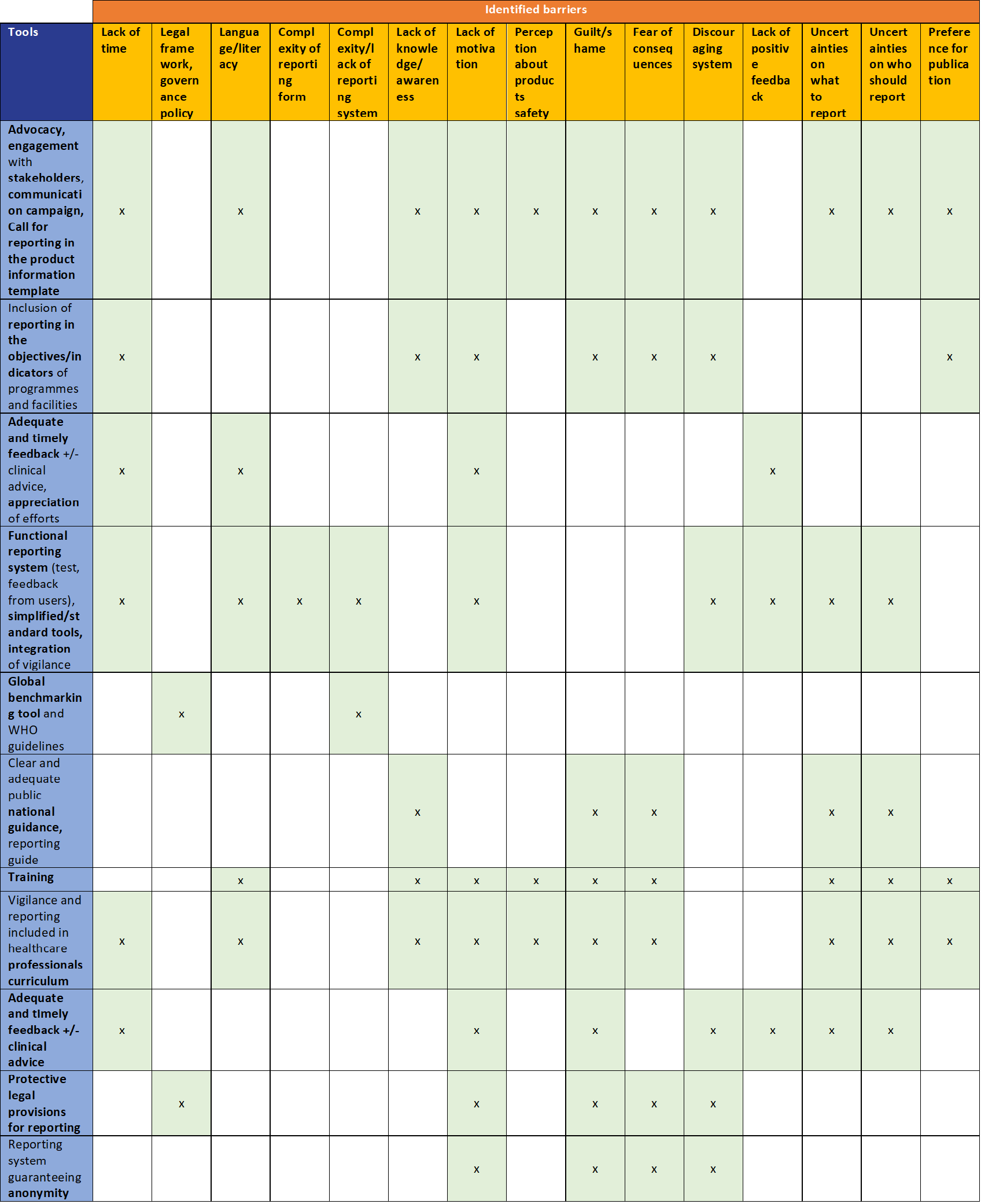
The following main barriers to reporting in the region were identified:
1.Lack of time
2.Legal framework, governance and policy
3.Language/literacy as a barrier
4.Complexity of reporting form
5.Complexity/lack of reporting system
6.Lack of knowledge/ awareness/training
7.Lack of motivation
8.Perception about products safety
9.Guilt/shame
10.Fear of consequences
11.Discouraging system
12.Lack of positive feedback to reporters
13.Uncertainties on what to report
14.Uncertainties on who should report
15.Preference for publication rather than PV report
Strategy to stimulate reporting
Considering the importance to adapt possible solutions in the local context, the strategy focuses on three main pillars:
- Providing a list of tools which can be considered at the national level to increase reporting
- Provide a template to facilitate the initiation of a national strategy to increase reporting
- Indicators and regular follow up through SEARN.
Tools to address the identified barriers
Each country is encouraged to build a national strategy based on the identified tools and working with all relevant stakeholders. WHO and other partners can provide support to assist with these efforts.
Template for a national strategy to increase reporting
The following template was developed to facilitate the initiation of a national strategy to increase reporting:
References
References
Afaya, Agani, Kennedy Diema Konlan, and Hyunok Kim Do. 2021. “Improving Patient Safety through Identifying Barriers to Reporting Medication Administration Errors among Nurses: An Integrative Review.” BMC Health Services Research 21 (1): 1156. https://doi.org/10.1186/s12913-021-07187-5.
Al Dweik, Rania, Dawn Stacey, Dafna Kohen, and Sanni Yaya. 2017. “Factors Affecting Patient Reporting of Adverse Drug Reactions: A Systematic Review.” British Journal of Clinical Pharmacology 83 (4): 875–83. https://doi.org/10.1111/bcp.13159.
Alharf, Adel, Nasser Alqahtani, Ghazi Saeed, Ali Alshahrani, Mubarak Alshahrani, Nasser Aljasser, Mohammed Alquwaizani, and Saleh Bawazir. 2018. “Saudi Vigilance Program: Challenges and Lessons Learned.” Saudi Pharmaceutical Journal 26 (3): 388–95. https://doi.org/10.1016/j.jsps.2018.01.002.
Aljabari, Salim, and Zuhal Kadhim. 2021. “Common Barriers to Reporting Medical Errors.” Edited by Sylvia H. Hsu. The Scientific World Journal 2021 (June): 1–8. https://doi.org/10.1155/2021/6494889.
Alomar, Muaed, Ali M Tawfiq, Nageeb Hassan, and Subish Palaian. 2020. “Post Marketing Surveillance of Suspected Adverse Drug Reactions through Spontaneous Reporting: Current Status, Challenges and the Future.” Therapeutic Advances in Drug Safety 11 (January): 204209862093859. https://doi.org/10.1177/2042098620938595.
Alsohime, F., Temsah, M.-H., Hasan, G., Al-Eyadhy, A., Gulman, S., Issa, H., Alsohime, O., 2019. Reporting adverse events related to medical devices: A single center experience from a tertiary academic hospital. PLoS ONE 14, e0224233. https://doi.org/10.1371/journal.pone.0224233
Alves, Michelle de Fatima Tavares, Denise Siqueira de Carvalho, and Guilherme Souza Cavalcanti de Albuquerque. 2019. “Motivos Para a Não Notificação de Incidentes de Segurança Do Paciente Por Profissionais de Saúde: Revisão Integrativa.” Ciência & Saúde Coletiva 24 (8): 2895–2908. https://doi.org/10.1590/1413-81232018248.23912017.
Arnal-Velasco, Daniel, and Paul Barach. 2021. “Anaesthesia and Perioperative Incident Reporting Systems: Opportunities and Challenges.” Best Practice & Research Clinical Anaesthesiology 35 (1): 93–103. https://doi.org/10.1016/j.bpa.2020.04.013.
Asma Ben Cheikh, Hela Ghali, Emna Mziou, Sana Bhiri, Mariem Ghribi, Nihel Haddad, Saloua Trabelsi, Selwa Khefacha, Mohamed Ben Rejeb, Houyem Said Latiri, 2022. Case Analysis of an Adverse Event Related to Treatments of Medical Devices According to ALARM Method. World Journal of Surgery and Surgical Research 5.
Balan, Shamala. 2021. “Knowledge, Attitude and Practice of Malaysian Healthcare Professionals toward Adverse Drug Reaction Reporting: A Systematic Review.” International Journal of Pharmacy Practice 29 (4): 308–20. https://doi.org/10.1093/ijpp/riab030.
CIOMS Cumulative Pharmacovigilance Glossary, Geneva, Switzerland: Council for International Organizations of Medical Sciences (CIOMS), 2021.
Crestan, Diana, Marta Paulina Trojniak, Sara Francescon, Giulia Fornasier, and Paolo Baldo. 2020. “Pharmacovigilance of Anti-Cancer Medicines: Opportunities and Challenges.” Expert Opinion on Drug Safety 19 (7): 849–60. https://doi.org/10.1080/14740338.2020.1772751.
Fukushima, Ayako, Noha Iessa, Madhava Ram Balakrishnan, and Shanthi Narayan Pal. 2022. “Smartphone-Based Mobile Applications for Adverse Drug Reactions Reporting: Global Status and Country Experience.” BMC Medical Informatics and Decision Making 22 (1): 118. https://doi.org/10.1186/s12911-022-01832-7.
Fung, Wing Mei, Serena Siew Lin Koh, and Yeow Leng Chow. 2012. “Attitudes and Perceived Barriers Influencing Incident Reporting by Nurses and Their Correlation with Reported Incidents: A Systematic Review:” JBI Library of Systematic Reviews 10 (1): 1–65. https://doi.org/10.11124/jbisrir-2012-44.
Gildeeva, Geliya, and Andrey Belostotsky. 2017. “Pharmacovigilance in Russia: Current State of Affairs, Challenges, and Prospects.” Current Medical Research and Opinion 33 (12): 2161–66. https://doi.org/10.1080/03007995.2017.1336082.
Gong, Yang. 2022. “Challenges and Opportunities of Patient Safety Event Reporting.” In Studies in Health Technology and Informatics, edited by Thomas M. Deserno, Mostafa Haghi, and Najeeb Al-Shorbaji. IOS Press. https://doi.org/10.3233/SHTI220014.
Hamed, Moataz Mohamed Maamoun, and Stathis Konstantinidis. 2022. “Barriers to Incident Reporting among Nurses: A Qualitative Systematic Review.” Western Journal of Nursing Research 44 (5): 506–23. https://doi.org/10.1177/0193945921999449.
Khan, Zakir, Yusuf Karatas, Maria Auxiliadora Parreiras Martins, Shazia Jamshed, and Hazir Rahman. 2022. “Knowledge, Attitude, Practice and Barriers towards Pharmacovigilance and Adverse Drug Reactions Reporting among Healthcare Professionals in Turkey: A Systematic Review.” Current Medical Research and Opinion 38 (1): 145–54. https://doi.org/10.1080/03007995.2021.1997287.
Mei, Fung Wing, Serena Koh Siew Lin, and Chow Yeow Leng. 2010. “Attitudes and Perceived Barriers Influencing Incident Reporting by Nurses and Their Correlation with Reported Incidents: A Systematic Review:” JBI Database of Systematic Reviews and Implementation Reports 8 (Supplement): 1–26. https://doi.org/10.11124/01938924-201008341-00026.
Perez, Bianca, Stephen A. Knych, Sallie J. Weaver, Aaron Liberman, Eileen M. Abel, Dawn Oetjen, and Thomas T. H. Wan. 2014. “Understanding the Barriers to Physician Error Reporting and Disclosure: A Systemic Approach to a Systemic Problem.” Journal of Patient Safety 10 (1): 45–51. https://doi.org/10.1097/PTS.0b013e31829e4b68.
Pfeiffer, Y., T. Manser, and T. Wehner. 2010. “Conceptualising Barriers to Incident Reporting: A Psychological Framework.” BMJ Quality & Safety 19 (6): e60–e60. https://doi.org/10.1136/qshc.2008.030445.
Polisena, J., Gagliardi, A., Urbach, D., Clifford, T., Fiander, M., 2015. Factors that influence the recognition, reporting and resolution of incidents related to medical devices and other healthcare technologies: a systematic review. Syst Rev 4, 37. https://doi.org/10.1186/s13643-015-0028-0
Salehi, Tahmine, Naiemeh Seyedfatemi, Mohammad Saeed Mirzaee, Maryam Maleki, and Abbas Mardani. 2021. “Nurses’ Knowledge, Attitudes, and Practice in Relation to Pharmacovigilance and Adverse Drug Reaction Reporting: A Systematic Review.” Edited by Muhammad Hassham Hassan Bin Asad. BioMed Research International 2021 (April): 1–12. https://doi.org/10.1155/2021/6630404.
Sawaya, Jennifer, Amanda Champlain, Joel Cohen, and Mathew Avram. 2021. “Barriers to Reporting: Limitations of the Maude Database.” Dermatologic Surgery Publish Ahead of Print (February). https://doi.org/10.1097/DSS.0000000000002832.
Siewert, Bettina, Olga R. Brook, Suzanne Swedeen, Ronald L. Eisenberg, and Mary Hochman. 2019. “Overcoming Human Barriers to Safety Event Reporting in Radiology.” RadioGraphics 39 (1): 251–63. https://doi.org/10.1148/rg.2019180135.
Song, Haibo, Xiaojing Pei, Zuoxiang Liu, Chuanyong Shen, Jun Sun, Yuqin Liu, Lingyun Zhou, Feng Sun, and Xiaohe Xiao. 2022. “Pharmacovigilance in China: Evolution and Future Challenges.” British Journal of Clinical Pharmacology, March, bcp.15277. https://doi.org/10.1111/bcp.15277.
Thomas, R.A., Rajan Joseph, M., Castilloux, A., Moride, Y., 2021. Understanding reporting practices and perceptions of barriers in adverse events following immunisation surveillance: A cross–sectional survey of paediatricians in Kerala, India. Vaccine 39, 4678–4684. https://doi.org/10.1016/j.vaccine.2021.06.052
Vrbnjak, Dominika, Suzanne Denieffe, Claire O’Gorman, and Majda Pajnkihar. 2016. “Barriers to Reporting Medication Errors and near Misses among Nurses: A Systematic Review.” International Journal of Nursing Studies 63 (November): 162–78. https://doi.org/10.1016/j.ijnurstu.2016.08.019.
WHO. 2012. Global Vaccine Safety Blueprint. Geneva: World Health Organization. https://apps.who.int/iris/handle/10665/70919.
WHO. 2020. Global Vaccine Action Plan: Monitoring, Evaluation and Accountability: Secretariat Annual Report 2020. Geneva: World Health Organization. https://apps.who.int/iris/handle/10665/337433.
WHO. n.d. “Adverse Events Following Immunization (AEFI).” n.d. https://www.who.int/teams/regulation-prequalification/regulation-and-safety/pharmacovigilance/health-professionals-info/aefi.
World Health Organization. 2021. Global Vaccine Safety Blueprint 2.0 (GVSB2.0) 2021-2023. Geneva: World Health Organization. https://apps.who.int/iris/handle/10665/348966.
Zhao, Ying, Tiansheng Wang, Guangyao Li, and Shusen Sun. 2018. “Pharmacovigilance in China: Development and Challenges.” International Journal of Clinical Pharmacy 40 (4): 823–31. https://doi.org/10.1007/s11096-018-0693-x.
