Action Point 11 - Strategy to support integration of vigilance
Last update: 27/07/2023
Background
Vigilance is the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other possible medical product-related problems (WHO n.d.). Vigilance also has the responsibility to provide authoritative information to the public and the different stakeholders regarding the safety of medical products.
The WHO Guidance for post-market surveillance and market surveillance of medical devices, including in vitro diagnostics (World Health Organization 2020), further refers to the closely related concepts of:
- market surveillance, i.e. the activities carried out and measures taken by competent authorities (regulatory authorities) to check and ensure that devices comply with the requirements set out in the relevant legislation and do not endanger health, safety or any other aspect of public interest protection;
- Post market Surveillance: The systematic process [carried out by manufacturers] to collect and analyse experience gained from medical devices that have been placed on the market.
Such surveillance systems involve complex sets of actors (including healthcare professionals, patients, regulators, program managers, manufacturers, experts, etc.) settings, and processes. In the SEARN countries, the development of vigilance has often been associated with public health programmes, especially immunization programmes, before being expanded to other products.
While there is no widely accepted definition of health system integration (and no definition of vigilance integration), it generally intends to put the need of the patient or the population at the center (Dawda 2019). This principle opposes fragmentation and aims at creating coherence, cooperation, coordination, and information sharing.
Considering that integration of vigilance deserved to be further deepened, the Assembly of SEARN adopted on 8 June 2022 the SEARN Work Plan 2022-2023 which includes the action point 11, led by Working Group 3 (WG3) Vigilance: ‘Draft a strategy to support integration of vigilance’.
Scope and definitions
For the purpose of this document, the following definitions are used:
- Coordination: the act of making all the people involved in a plan or activity work together in an organized way (Cambridge Dictionary)
- Market surveillance (for medical devices): The activities carried out and measures taken by competent authorities (regulatory authorities) to check and ensure that devices comply with the requirements set out in the relevant legislation and do not endanger health, safety or any other aspect of public interest protection. (WHO 2020 Guidance for post-market surveillance and market surveillance of medical devices, including in vitro diagnostics)
- Market surveillance and control: Market surveillance and control function plays a crucial role in assuring medical products consumer safety since its objective is to ensure compliance of the products placed on the market with pre-set criteria for quality, safety and efficacy (i.e., verify compliance with marketing authorization and GXP guidelines). Market surveillance and control function activities are primarily concerned with four themes: (1) control of import activities, (2) prevention and detection of and response to substandard and falsified medical products, (3) market surveillance program for monitoring the quality of medical products throughout the supply chain, and (4) control of promotional, marketing and advertising activities. The aforementioned activities may or may not be undertaken by a single entity (e.g., organization, division, or department). (WHO Global Benchmarking Tool (GBT) for Evaluation of National Regulatory System of Medical Products, Revision VI)
- Pharmacovigilance: the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other medicine/vaccine related problem (WHO)
- Stakeholder: An individual, group or an organization that has an interest in the organization and delivery of health care. (WHO Global Benchmarking Tool (GBT) for Evaluation of National Regulatory System of Medical Products, Revision VI)
- Post-market surveillance (for medical devices): Systematic process [performed by the manufacturer] to collect and analyse experience gained from medical devices that have been placed on the market. (WHO 2020 Guidance for post-market surveillance and market surveillance of medical devices, including in vitro diagnostics)
- Vigilance integration: the continuous efforts of the different stakeholders of a common vigilance regulatory system (e.g. manufacturers, regulatory authorities, disease programmes, vigilance centres) to reach and maintain the level of coordination required to protect public health.
The scope of this document covers all medical products within the scope of SEARN, and includes pharmacovigilance, market surveillance and post-market surveillance for medical devices, and market surveillance and control of substandard and falsified product.
Objectives
To recommend practical solutions to optimize cooperation between stakeholders and ensure adequate and timely action are taken to minimize the risks.
Definition of Vigilance integration
In the context of this strategy, integration refers to the continuous efforts of the different stakeholders of a common vigilance regulatory system (e.g. manufacturers, regulatory authorities, disease programmes, vigilance centres) to reach and maintain the level of coordination required to protect public health.
Main stakeholders of the vigilance regulatory system(s)
Stakeholders at the national level
Acknowledging the diversity of vigilance regulatory systems within and between the Member States, there are likely important variations in the number, type, and responsibilities of the different stakeholders.
These actors may further be classified based on the scope and objectives of vigilance integration:
Critical stakeholders for vigilance integration
- Regional and national vigilance centers
- National regulatory authorities
- Public health programmes (e.g. Tuberculosis, Malaria, HIV, etc.) and immunization programmes at all geographic levels
- Manufacturers, marketing authorization holders, other economic operators (e.g. importers, wholesalers, authorized representatives, etc.)
- National Control Laboratory
Stakeholders which may be considered for vigilance integration (opportunity)
- Clinical centers of excellence, clinical research organisations, universities
- Other departments of the Ministry of Health
- Center for traditional medicine
- Hospital safety committees
- Poison control centers
- Patients and consumer associations
- Professional associations (e.g. medical doctors, pharmacists, nurses, etc.)
- Relevant Non-Governmental Organizations
- Donors
Important stakeholders beyond the objective of integration
- Patients, caretakers, users, healthcare professionals and allied professions
- Media
Stakeholders at the International level
International and regional vigilance integration is driven by the assumption that what is observed in another population may happen also in the country’s population, unless demonstrated otherwise. Further, the larger a population is, the more power pharmacovigilance has to detect issues, and aggregating the data from different countries can be a way to increase the chances of detecting issues relevant to all countries. Finally, integration may also be a means to learn from the experience of other countries, including regarding effective risk minimisation measures.
The WHO Programme for International Drug Monitoring (PIDM) is the global vigilance platform to share the vision of safer use of medicinal products. This is an example of platforms aiming for international integration of vigilance through sharing ICSR for medicinal products into the WHO global database of reported adverse events (VigiBase). The shared ICSRs are accessible by the PIDM Members WHO and the WHO Collaborating Centres (WHO-CCs) according to the data access policy. Analysis outputs could be disseminated through different channels such as the periodic WHO Pharmaceutical Newsletters. The analyses are supported by the WHO Collaborating Centre for International Drug Monitoring (UMC). Regional networks, such as SEARN, may also offer platform for integration of vigilance.
WHO’s Global Surveillance and Monitoring System collects incidents for in vitro diagnostic medical devices (IVDs) and certain medical devices that are WHO approved (prequalified, emergency use listed or otherwise recommended by WHO). This is primarily to ensure that the relevant WHO requirements have been met when incidents are reported by device user to manufacturer with respect to any correction, corrective and preventive action taken.
Integration of Vigilance
Benefits
Many benefits are achieved only when all actors at all levels closely collaborate. The integration of vigilance provides multiples opportunities to stakeholders to avoid redundancy, access the information they require and optimise the decisions taken. For example, collecting and analysing safety information within each stakeholder may limit the detection of safety signals of medicines and vaccines due to insufficient quantity of data. Collaborating and gathering information facilitate the detection of safety signals and increase the probability of detecting rare adverse reactions from the larger data. In addition, entities with limited capacity to perform core vigilance activities will take advantage of vigilance integration for various aspects such as human and financial resources, expertise available for fulfilling activities and availability of proper training etc.
Breaking barriers and building bridges. Integrated vigilance is our strength.
Levels of vigilance integration
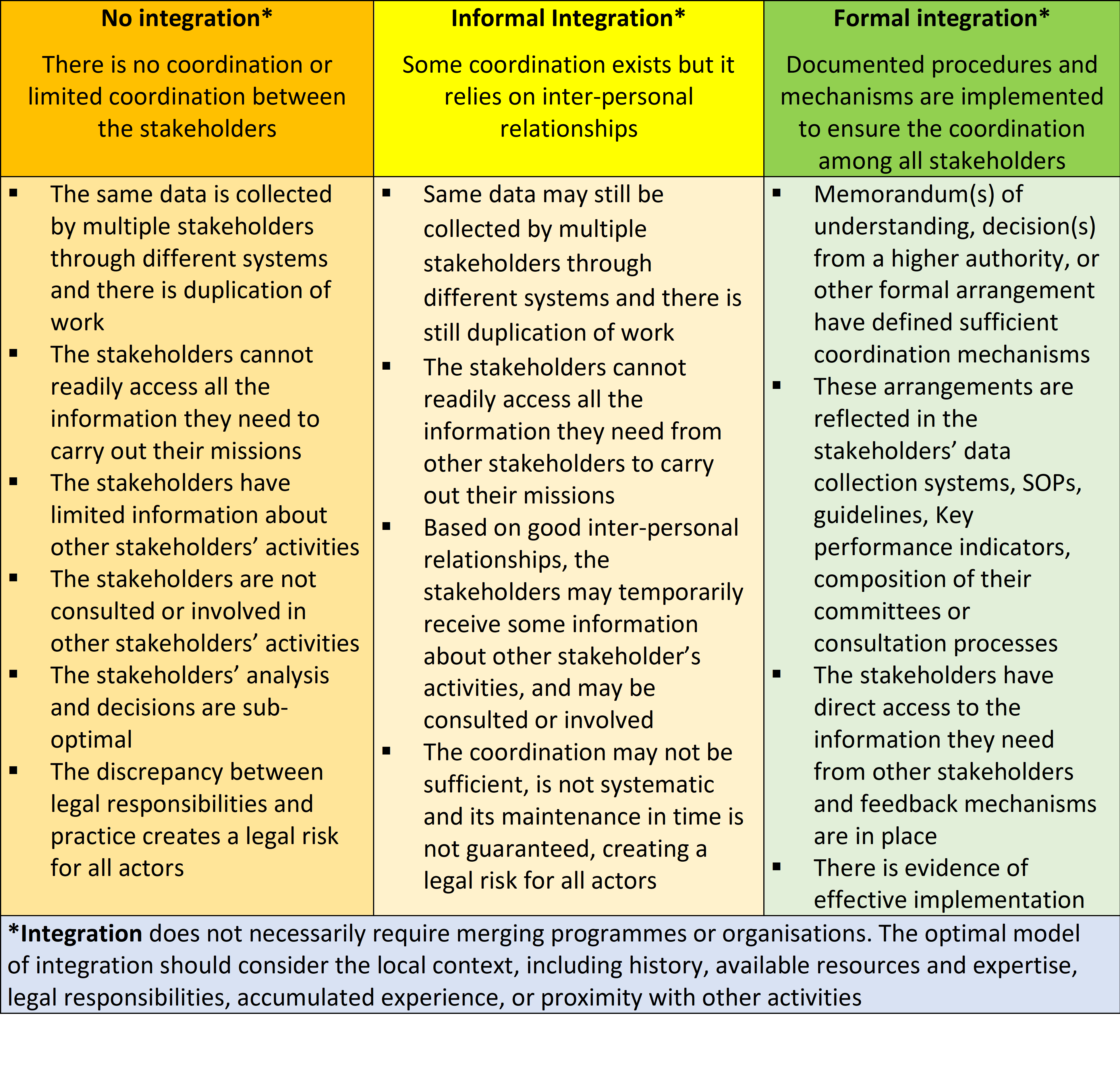
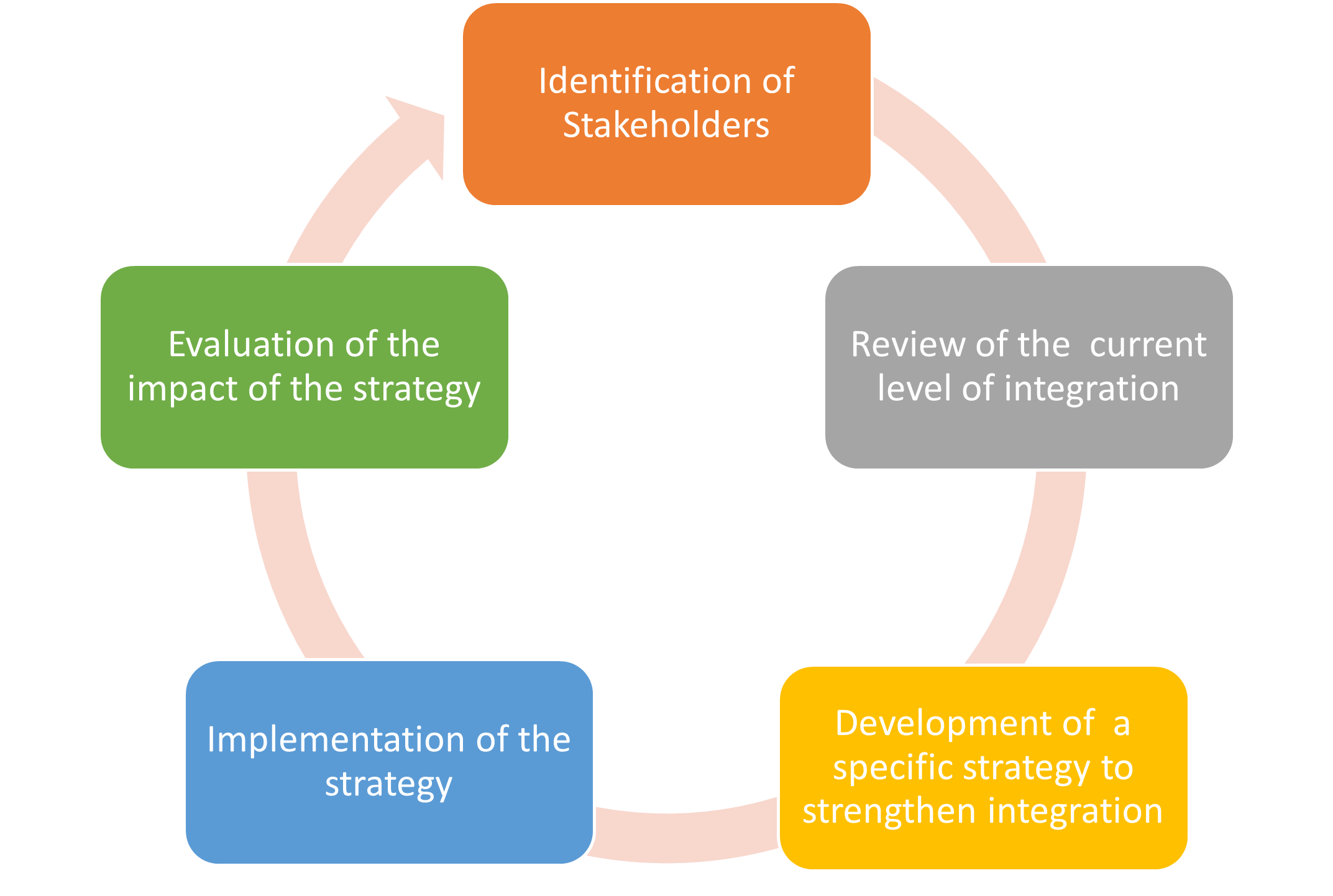
Process of vigilance integration
The key steps to develop vigilance integration are presented below:
To assist in this process, the following table may be used:
Challenges to integration and practical solutions
The following challenges to vigilance integration between the critical stakeholders may be identified (non-exhaustive list):

Dissemination
The following PowerPoint template was developed as advocacy material to support discussions between stakeholders on vigilance integration:
References
References
Dawda, Paresh. 2019. “Integrated Healthcare: The Past, Present and Future.” Integrated Healthcare Journal 1 (1): e000001. https://doi.org/10.1136/ihj-2019-000001.
WHO. n.d. “Pharmacovigilance.” Accessed October 24, 2022. https://www.who.int/teams/regulation-prequalification/regulation-and-safety/pharmacovigilance.
World Health Organization. 2020. Guidance for Post-Market Surveillance and Market Surveillance of Medical Devices, Including in Vitro Diagnostics. Geneva: World Health Organization. https://apps.who.int/iris/handle/10665/337551.
